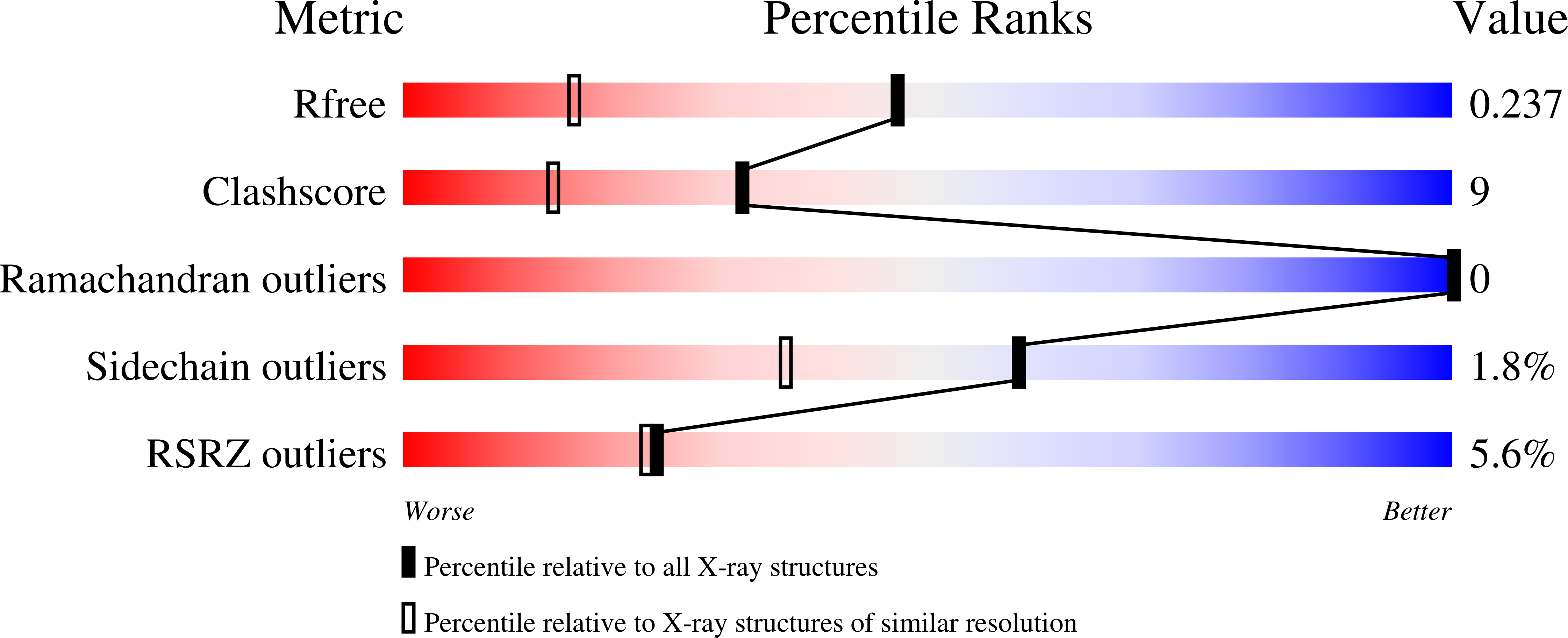
Kinetic and structural characterization of bacterial glutaminyl cyclases from Zymomonas mobilis and Myxococcus xanthus
Carrillo, D.R., Parthier, C., Janckel, N., Grandke, J., Stelter, M., Schilling, S., Boehme, M., Neumann, P., Wolf, R., Demuth, H.U., Stubbs, M.T., Rahfeld, J.U.(2010) Biol Chem 391: 1419-1428
- PubMed: 20868223
- DOI: https://doi.org/10.1515/BC.2010.130
- Primary Citation of Related Structures:
3NOK, 3NOL, 3NOM - PubMed Abstract:
Although enzymes responsible for the cyclization of amino-terminal glutamine residues are present in both plant and mammal species, none have yet been characterized in bacteria. Based on low sequence homologies to plant glutaminyl cyclases (QCs), we cloned the coding sequences of putative microbial QCs from Zymomonas mobilis (ZmQC) and Myxococcus xanthus (MxQC). The two recombinant enzymes exhibited distinct QC activity, with specificity constants k(cat)/K(m) of 1.47¡À0.33 mm?? s?? (ZmQC) and 142¡À32.7 mm?? s?? (MxQC) towards the fluorescent substrate glutamine-7-amino-4-methyl-coumarine. The measured pH-rate profile of the second order rate constant displayed an interesting deviation towards the acidic limb of the pH chart in the case of ZmQC, whereas MxQC showed maximum activity in the mild alkaline pH range. Analysis of the enzyme variants ZmQCGlu??Gln and MxQCGln??Glu show that the exchanged residues play a significant role in the pH behaviour of the respective enzymes. In addition, we determined the three dimensional crystal structures of both enzymes. The tertiary structure is defined by a five-bladed ¦Â-propeller anchored by a core cation. The structures corroborate the putative location of the active site and confirm the proposed relation between bacterial and plant glutaminyl cyclases.
Organizational Affiliation:
Probiodrug AG, Halle/Saale, Germany.