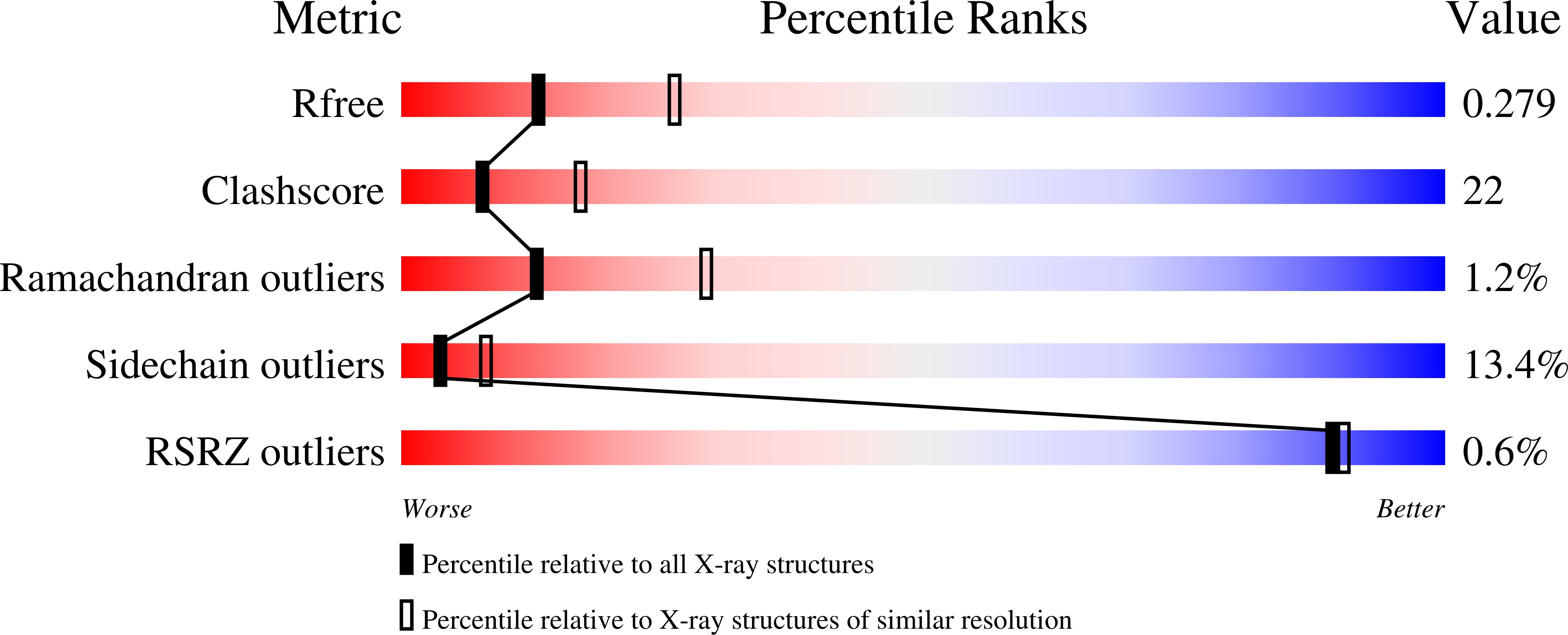
Active mutants of the TCR-mediated p38alpha alternative activation site show changes in the phosphorylation lip and DEF site formation.
Tzarum, N., Diskin, R., Engelberg, D., Livnah, O.(2011) J Mol Biology 405: 1154-1169
- PubMed: 21146537
- DOI: https://doi.org/10.1016/j.jmb.2010.11.023
- Primary Citation of Related Structures:
3OD6, 3ODY, 3ODZ, 3OEF - PubMed Abstract:
The p38¦Á mitogen-activated protein kinase is commonly activated by dual (Thr and Tyr) phosphorylation catalyzed by mitogen-activated protein kinase kinases. However, in T-cells, upon stimulation of the T-cell receptor, p38¦Á is activated via an alternative pathway, involving its phosphorylation by zeta-chain-associated protein kinase 70 on Tyr323, distal from the phosphorylation lip. Tyr323-phosphorylated p38¦Á is autoactivated, resulting in monophosphorylation of Thr180. The conformational changes induced by pTyr323 mediating autoactivation are not known. The lack of pTyr323 p38¦Á for structural studies promoted the search for Tyr323 mutations that may functionally emulate its effect when phosphorylated. Via a comprehensive mutagenesis of Tyr323, we identified mutations that rendered the kinase intrinsically active and others that displayed no activity. Crystallographic studies of selected active (p38¦Á(Y323Q), p38¦Á(Y323T), and p38¦Á(Y323R)) and inactive (p38¦Á(Y323F)) mutants revealed that substantial changes in interlobe orientation, extended conformation of the activation loop, and formation of substrate docking DEF site (docking site for extracellular signal-regulated kinase FXF) interaction pocket are associated with p38¦Á activation.
Organizational Affiliation:
Department of Biological Chemistry, Alexander Silberman Institute of Life Sciences, Hebrew University of Jerusalem, Jerusalem 91904, Israel.