Biochemical and Structural Studies of Uncharacterized Protein PA0743 from Pseudomonas aeruginosa Revealed NAD+-dependent L-Serine Dehydrogenase.
Tchigvintsev, A., Singer, A., Brown, G., Flick, R., Evdokimova, E., Tan, K., Gonzalez, C.F., Savchenko, A., Yakunin, A.F.(2012) J Biological Chem 287: 1874-1883
- PubMed: 22128181
- DOI: https://doi.org/10.1074/jbc.M111.294561
- Primary Citation of Related Structures:
3OBB, 3Q3C - PubMed Abstract:
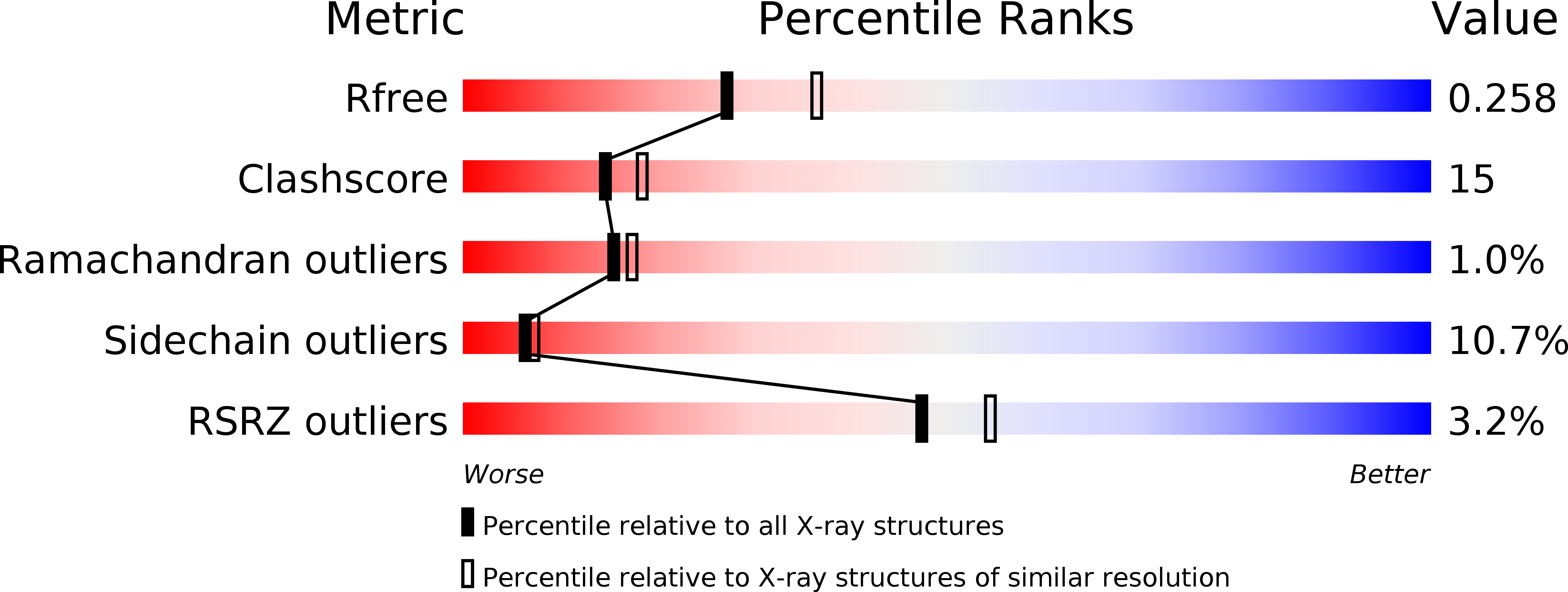
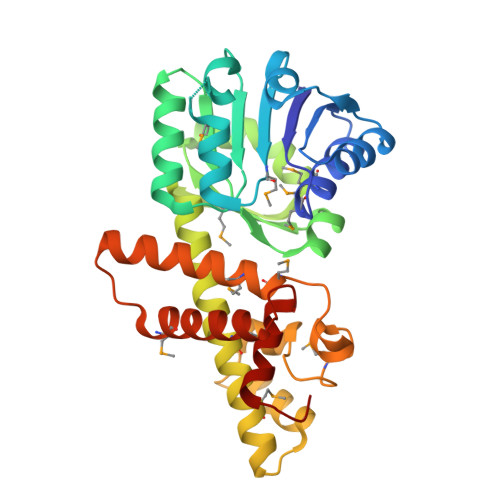
The ¦Â-hydroxyacid dehydrogenases form a large family of ubiquitous enzymes that catalyze oxidation of various ¦Â-hydroxy acid substrates to corresponding semialdehydes. Several known enzymes include ¦Â-hydroxyisobutyrate dehydrogenase, 6-phosphogluconate dehydrogenase, 2-(hydroxymethyl)glutarate dehydrogenase, and phenylserine dehydrogenase, but the vast majority of ¦Â-hydroxyacid dehydrogenases remain uncharacterized. Here, we demonstrate that the predicted ¦Â-hydroxyisobutyrate dehydrogenase PA0743 from Pseudomonas aeruginosa catalyzes an NAD(+)-dependent oxidation of l-serine and methyl-l-serine but exhibits low activity against ¦Â-hydroxyisobutyrate. Two crystal structures of PA0743 were solved at 2.2-2.3-? resolution and revealed an N-terminal Rossmann fold domain connected by a long ¦Á-helix to the C-terminal all-¦Á domain. The PA0743 apostructure showed the presence of additional density modeled as HEPES bound in the interdomain cleft close to the predicted catalytic Lys-171, revealing the molecular details of the PA0743 substrate-binding site. The structure of the PA0743-NAD(+) complex demonstrated that the opposite side of the enzyme active site accommodates the cofactor, which is also bound near Lys-171. Site-directed mutagenesis of PA0743 emphasized the critical role of four amino acid residues in catalysis including the primary catalytic residue Lys-171. Our results provide further insight into the molecular mechanisms of substrate selectivity and activity of ¦Â-hydroxyacid dehydrogenases.
Organizational Affiliation:
Department of Chemical Engineering, University of Toronto, Toronto, Ontario M5G 1L6, Canada.