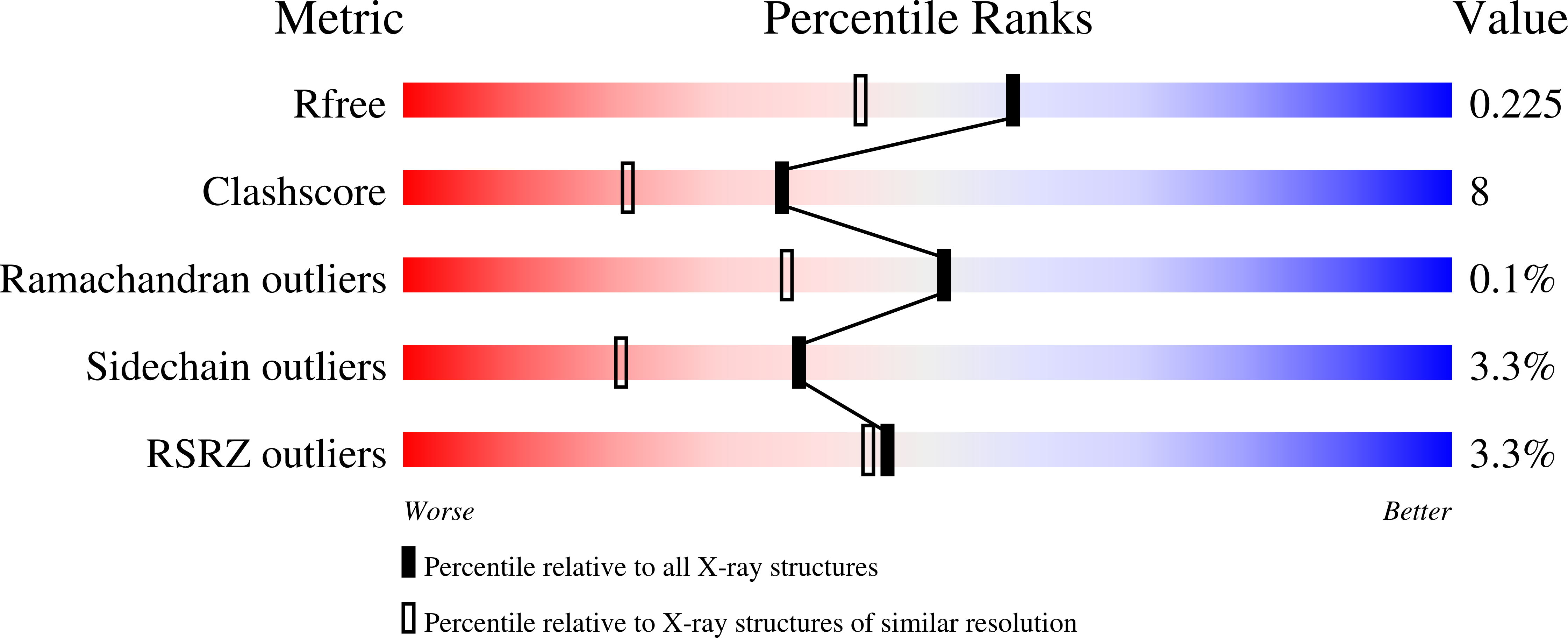
Probing oxygen activation sites in two flavoprotein oxidases using chloride as an oxygen surrogate.
Kommoju, P.R., Chen, Z.W., Bruckner, R.C., Mathews, F.S., Jorns, M.S.(2011) Biochemistry 50: 5521-5534
- PubMed: 21568312
- DOI: https://doi.org/10.1021/bi200388g
- Primary Citation of Related Structures:
3QSE, 3QSM, 3QSS, 3QVP, 3QVR - PubMed Abstract:
A single basic residue above the si-face of the flavin ring is the site of oxygen activation in glucose oxidase (GOX) (His516) and monomeric sarcosine oxidase (MSOX) (Lys265). Crystal structures of both flavoenzymes exhibit a small pocket at the oxygen activation site that might provide a preorganized binding site for superoxide anion, an obligatory intermediate in the two-electron reduction of oxygen. Chloride binds at these polar oxygen activation sites, as judged by solution and structural studies. First, chloride forms spectrally detectable complexes with GOX and MSOX. The protonated form of His516 is required for tight binding of chloride to oxidized GOX and for rapid reaction of reduced GOX with oxygen. Formation of a binary MSOXˇ¤chloride complex requires Lys265 and is not observed with Lys265Met. Binding of chloride to MSOX does not affect the binding of a sarcosine analogue (MTA, methylthioactetate) above the re-face of the flavin ring. Definitive evidence is provided by crystal structures determined for a binary MSOXˇ¤chloride complex and a ternary MSOXˇ¤chlorideˇ¤MTA complex. Chloride binds in the small pocket at a position otherwise occupied by a water molecule and forms hydrogen bonds to four ligands that are arranged in approximate tetrahedral geometry: Lys265:NZ, Arg49:NH1, and two water molecules, one of which is hydrogen bonded to FAD:N5. The results show that chloride (i) acts as an oxygen surrogate, (ii) is an effective probe of polar oxygen activation sites, and (iii) provides a valuable complementary tool to the xenon gas method that is used to map nonpolar oxygen-binding cavities.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Drexel University College of Medicine, Philadelphia, Pennsylvania 19102, USA.