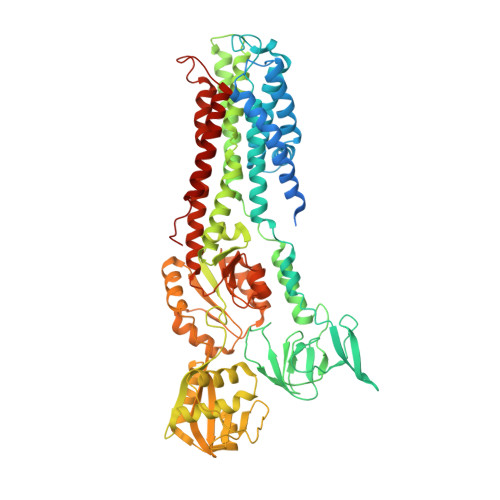
Crystal structure of a copper-transporting PIB-type ATPase.
Gourdon, P., Liu, X.Y., Skjorringe, T., Morth, J.P., Moller, L.B., Pedersen, B.P., Nissen, P.(2011) Nature 475: 59-64
- PubMed: 21716286
- DOI: https://doi.org/10.1038/nature10191
- Primary Citation of Related Structures:
3RFU - PubMed Abstract:
Heavy-metal homeostasis and detoxification is crucial for cell viability. P-type ATPases of the class IB (PIB) are essential in these processes, actively extruding heavy metals from the cytoplasm of cells. Here we present the structure of a PIB-ATPase, a Legionella pneumophila CopA Cu(+)-ATPase, in a copper-free form, as determined by X-ray crystallography at 3.2 ? resolution. The structure indicates a three-stage copper transport pathway involving several conserved residues. A PIB-specific transmembrane helix kinks at a double-glycine motif displaying an amphipathic helix that lines a putative copper entry point at the intracellular interface. Comparisons to Ca(2+)-ATPase suggest an ATPase-coupled copper release mechanism from the binding sites in the membrane via an extracellular exit site. The structure also provides a framework to analyse missense mutations in the human ATP7A and ATP7B proteins associated with Menkes' and Wilson's diseases.
Organizational Affiliation:
Centre for Membrane Pumps in Cells and Disease-PUMPKIN, Danish National Research Foundation, Aarhus University, Department of Molecular Biology, Gustav Wieds Vej 10C, DK-8000 Aarhus C, Denmark.