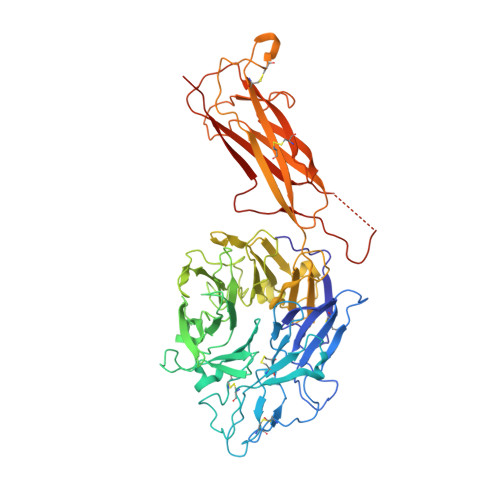
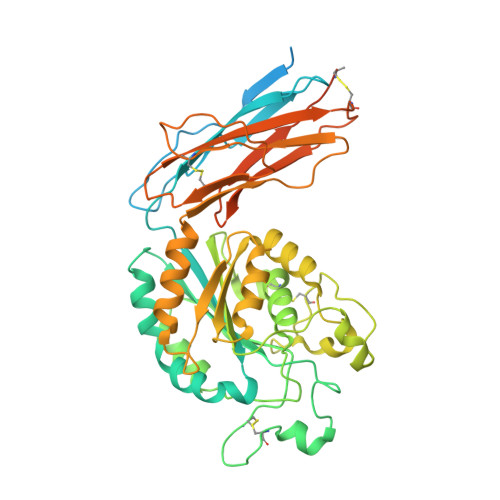
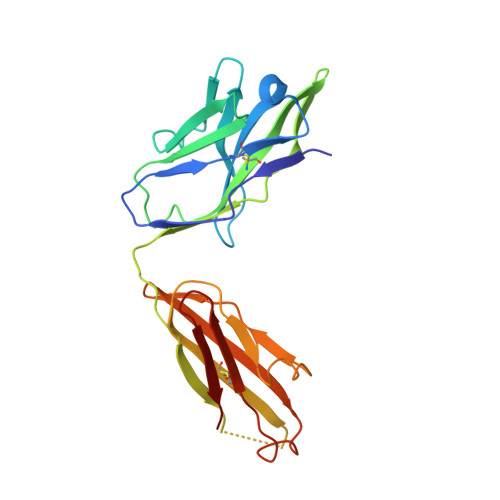
Structural specializations of a4b7, an Integrin that Mediates Rolling Adhesion
Yu, Y., Zhu, J., Mi, L.Z., Walz, T., Sun, H., Chen, J., Springer, T.A.(2012) J Cell Biol 196: 131-146
- PubMed: 22232704
- DOI: https://doi.org/10.1083/jcb.201110023
- Primary Citation of Related Structures:
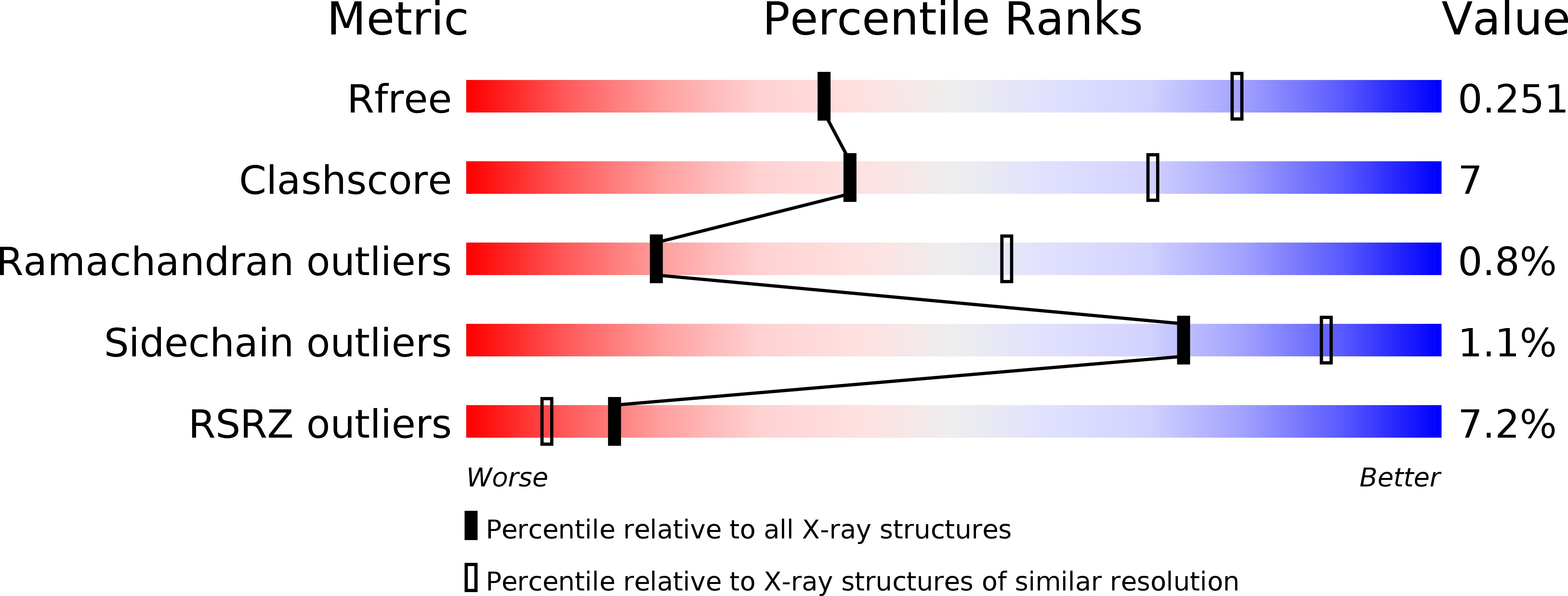
3V4P, 3V4V - PubMed Abstract:
The lymphocyte homing receptor integrin ¦Á(4)¦Â(7) is unusual for its ability to mediate both rolling and firm adhesion. ¦Á(4)¦Â(1) and ¦Á(4)¦Â(7) are targeted by therapeutics approved for multiple sclerosis and Crohn's disease. Here, we show by electron microscopy and crystallography how two therapeutic Fabs, a small molecule (RO0505376), and mucosal adhesion molecule-1 (MAdCAM-1) bind ¦Á(4)¦Â(7). A long binding groove at the ¦Á(4)-¦Â(7) interface for immunoglobulin superfamily domains differs in shape from integrin pockets that bind Arg-Gly-Asp motifs. RO0505376 mimics an Ile/Leu-Asp motif in ¦Á(4) ligands, and orients differently from Arg-Gly-Asp mimics. A novel auxiliary residue at the metal ion-dependent adhesion site in ¦Á(4)¦Â(7) is essential for binding to MAdCAM-1 in Mg(2+) yet swings away when RO0505376 binds. A novel intermediate conformation of the ¦Á(4)¦Â(7) headpiece binds MAdCAM-1 and supports rolling adhesion. Lack of induction of the open headpiece conformation by ligand binding enables rolling adhesion to persist until integrin activation is signaled.
Organizational Affiliation:
Department of Biological Chemistry and Molecular Pharmacology, Immune Disease Institute and Children's Hospital, Boston, MA 02115, USA.