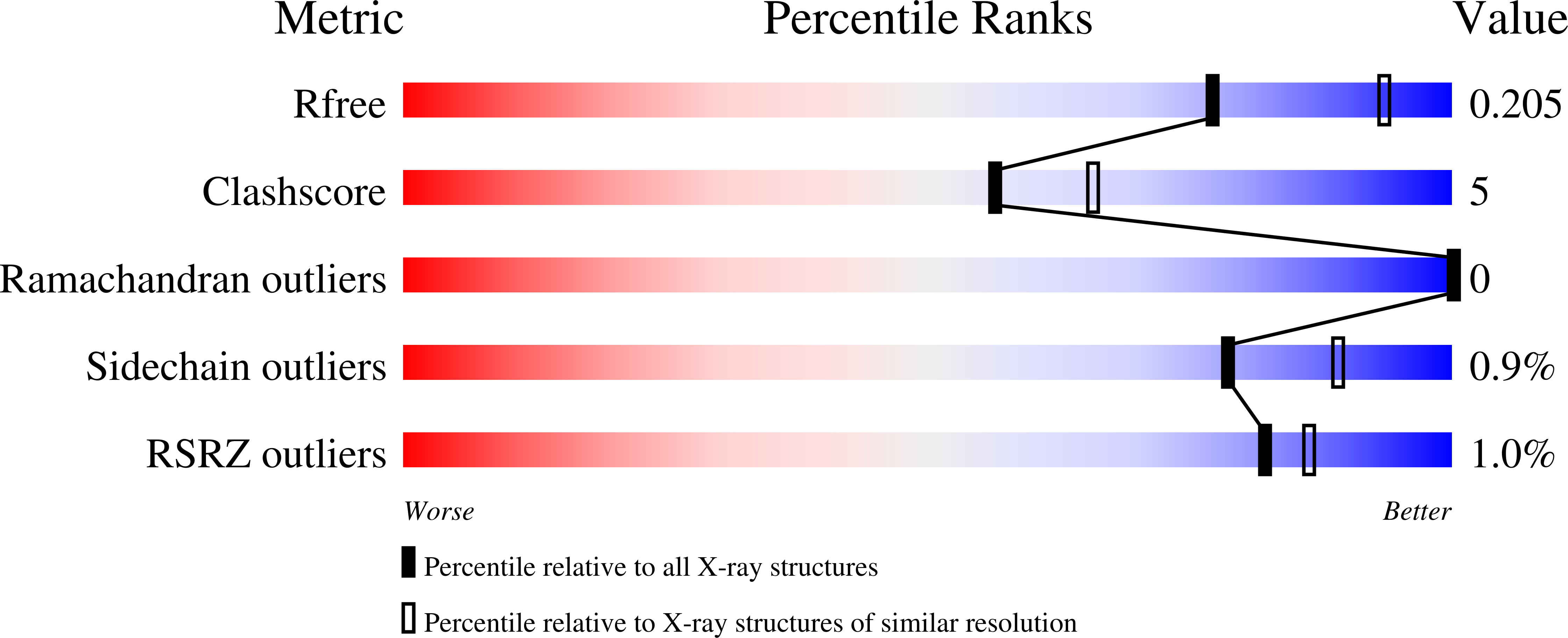
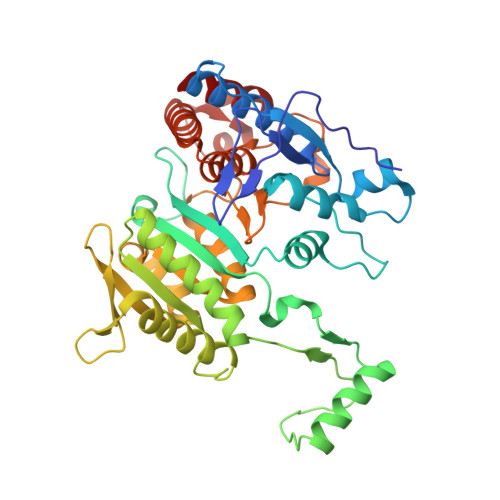
Induced Fit and the Catalytic Mechanism of Isocitrate Dehydrogenase.
Goncalves, S., Miller, S.P., Carrondo, M.A., Dean, A.M., Matias, P.M.(2012) Biochemistry 51: 7098
- PubMed: 22891681
- DOI: https://doi.org/10.1021/bi300483w
- Primary Citation of Related Structures:
4AJ3, 4AJA, 4AJB, 4AJC, 4AJR, 4AJS - PubMed Abstract:
NADP(+) dependent isocitrate dehydrogenase (IDH; EC 1.1.1.42) belongs to a large family of ¦Á-hydroxyacid oxidative ¦Â-decarboxylases that catalyze similar three-step reactions, with dehydrogenation to an oxaloacid intermediate preceding ¦Â-decarboxylation to an enol intermediate followed by tautomerization to the final ¦Á-ketone product. A comprehensive view of the induced fit needed for catalysis is revealed on comparing the first "fully closed" crystal structures of a pseudo-Michaelis complex of wild-type Escherichia coli IDH (EcoIDH) and the "fully closed" reaction product complex of the K100M mutant with previously obtained "quasi-closed" and "open" conformations. Conserved catalytic residues, binding the nicotinamide ring of NADP(+) and the metal-bound substrate, move as rigid bodies during domain closure by a hinge motion that spans the central ¦Â-sheet in each monomer. Interactions established between Thr105 and Ser113, which flank the "phosphorylation loop", and the nicotinamide mononucleotide moiety of NADP(+) establish productive coenzyme binding. Electrostatic interactions of a Lys100-Leu103-Asn115-Glu336 tetrad play a pivotal role in assembling a catalytically competent active site. As predicted, Lys230* is positioned to deprotonate/reprotonate the ¦Á-hydroxyl in both reaction steps and Tyr160 moves into position to protonate C3 following ¦Â-decarboxylation. A proton relay from the catalytic triad Tyr160-Asp307-Lys230* connects the ¦Á-hydroxyl of isocitrate to the bulk solvent to complete the picture of the catalytic mechanism.
Organizational Affiliation:
Instituto de Tecnologia Qu¨ªmica e Biol¨®gica, Universidade Nova de Lisboa, Apartado 127, 2780 Oeiras Portugal.