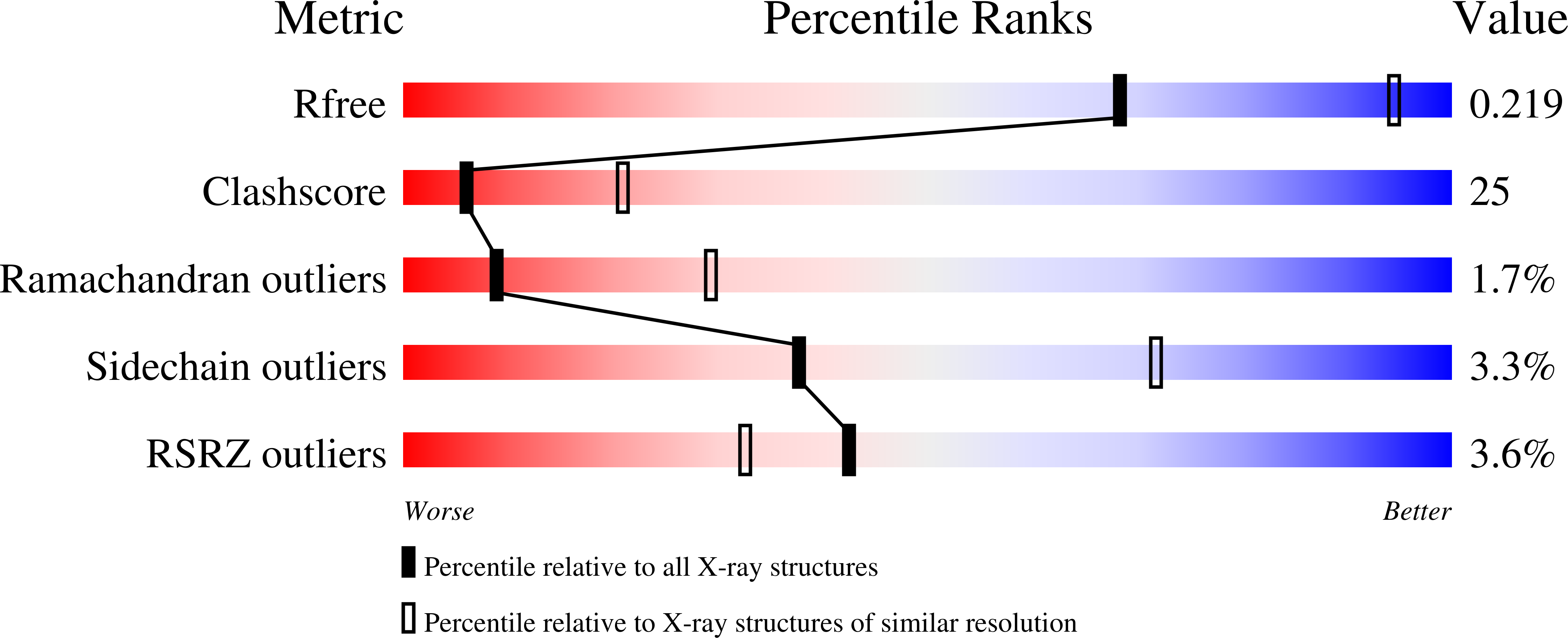
Insights into BAY 60-2770 Activation and S-Nitrosylation-Dependent Desensitization of Soluble Guanylyl Cyclase via Crystal Structures of Homologous Nostoc H-NOX Domain Complexes.
Kumar, V., Martin, F., Hahn, M.G., Schaefer, M., Stamler, J.S., Stasch, J.P., van den Akker, F.(2013) Biochemistry 52: 3601-3608
- PubMed: 23614626
- DOI: https://doi.org/10.1021/bi301657w
- Primary Citation of Related Structures:
4IAE, 4IAH, 4IAM - PubMed Abstract:
The soluble guanylyl cyclase (sGC) is an important receptor for nitric oxide (NO). Nitric oxide activates sGC several hundred fold to generate cGMP from GTP. Because of sGC's salutary roles in cardiovascular physiology, it has received substantial attention as a drug target. The heme domain of sGC is key to its regulation as it not only contains the NO activation site but also harbors sites for NO-independent sGC activators as well an S-nitrosylation site (¦Â1 C122) involved in desensitization. Here we report the crystal structure of the activator BAY 60-2770 bound to the Nostoc H-NOX domain that is homologous to sGC. The structure reveals that BAY 60-2770 has displaced the heme and acts as a heme mimetic via carboxylate-mediated interactions with the conserved YxSxR motif as well as hydrophobic interactions. Comparisons with the previously determined BAY 58-2667 bound structure reveal that BAY 60-2770 is more ordered in its hydrophobic tail region. sGC activity assays demonstrate that BAY 60-2770 has about 10% higher fold maximal stimulation compared to BAY 58-2667. S-Nitrosylation of the BAY 60-2770 substituted Nostoc H-NOX domain causes subtle changes in the vicinity of the S-nitrosylated C122 residue. These shifts could impact the adjacent YxSxR motif and ¦ÁF helix and as such potentially inhibit either heme incorporation or NO-activation of sGC and thus provide a structural basis for desensitization.
Organizational Affiliation:
Department of Biochemistry, Case Western Reserve University , Cleveland, Ohio 44106, United States.