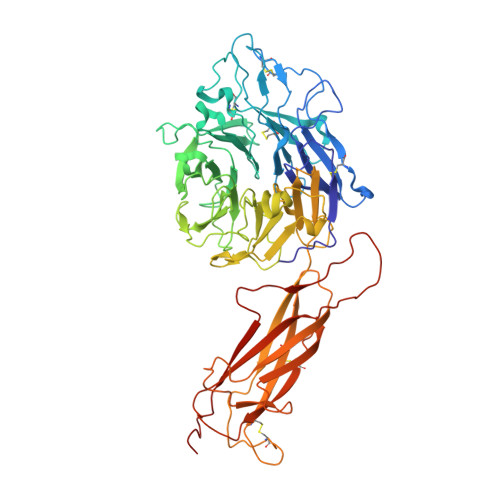
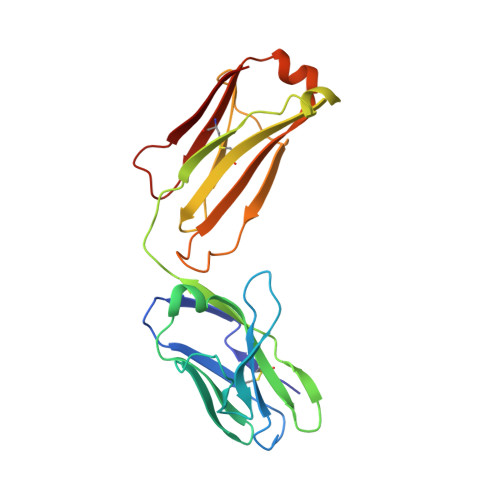
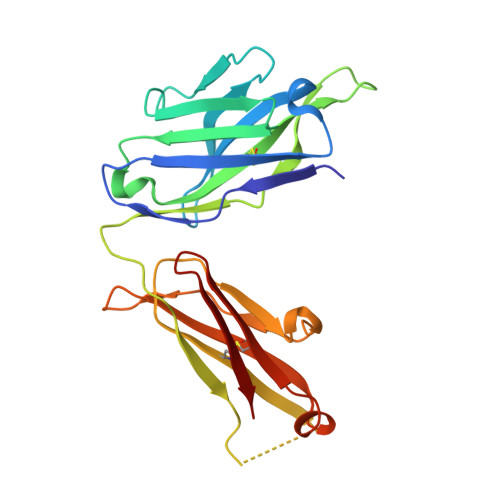
How natalizumab binds and antagonizes alpha 4 integrins.
Yu, Y., Schurpf, T., Springer, T.A.(2013) J Biol Chem 288: 32314-32325
- PubMed: 24047894
- DOI: https://doi.org/10.1074/jbc.M113.501668
- Primary Citation of Related Structures:
4IRZ - PubMed Abstract:
Natalizumab antibody to ¦Á4-integrins is used in therapy of multiple sclerosis and Crohn's disease. A crystal structure of the Fab bound to an ¦Á4 integrin ¦Â-propeller and thigh domain fragment shows that natalizumab recognizes human-mouse differences on the circumference of the ¦Â-propeller domain. The epitope is adjacent to but outside of a ligand-binding groove formed at the interface with the ¦Â-subunit ¦ÂI domain and shows no difference in structure when bound to Fab. Competition between Fab and the ligand vascular cell adhesion molecule (VCAM) for binding to cell surface ¦Á4¦Â1 shows noncompetitive antagonism. In agreement, VCAM docking models suggest that binding of domain 1 of VCAM to ¦Á4-integrins is unimpeded by the Fab, and that bound Fab requires a change in orientation between domains 1 and 2 of VCAM for binding to ¦Á4¦Â1. Mapping of species-specific differences onto ¦Á4¦Â1 and ¦Á4¦Â7 shows that their ligand-binding sites are highly conserved. Skewing away from these conserved regions of the epitopes recognized by current therapeutic function-blocking antibodies has resulted in previously unanticipated mechanisms of action.
Organizational Affiliation:
From the Program in Cellular and Molecular Medicine, Children's Hospital Boston and Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, Massachusetts 02115.