A radical transfer pathway in spore photoproduct lyase.
Yang, L., Nelson, R.S., Benjdia, A., Lin, G., Telser, J., Stoll, S., Schlichting, I., Li, L.(2013) Biochemistry 52: 3041-3050
- PubMed: 23607538
- DOI: https://doi.org/10.1021/bi3016247
- Primary Citation of Related Structures:
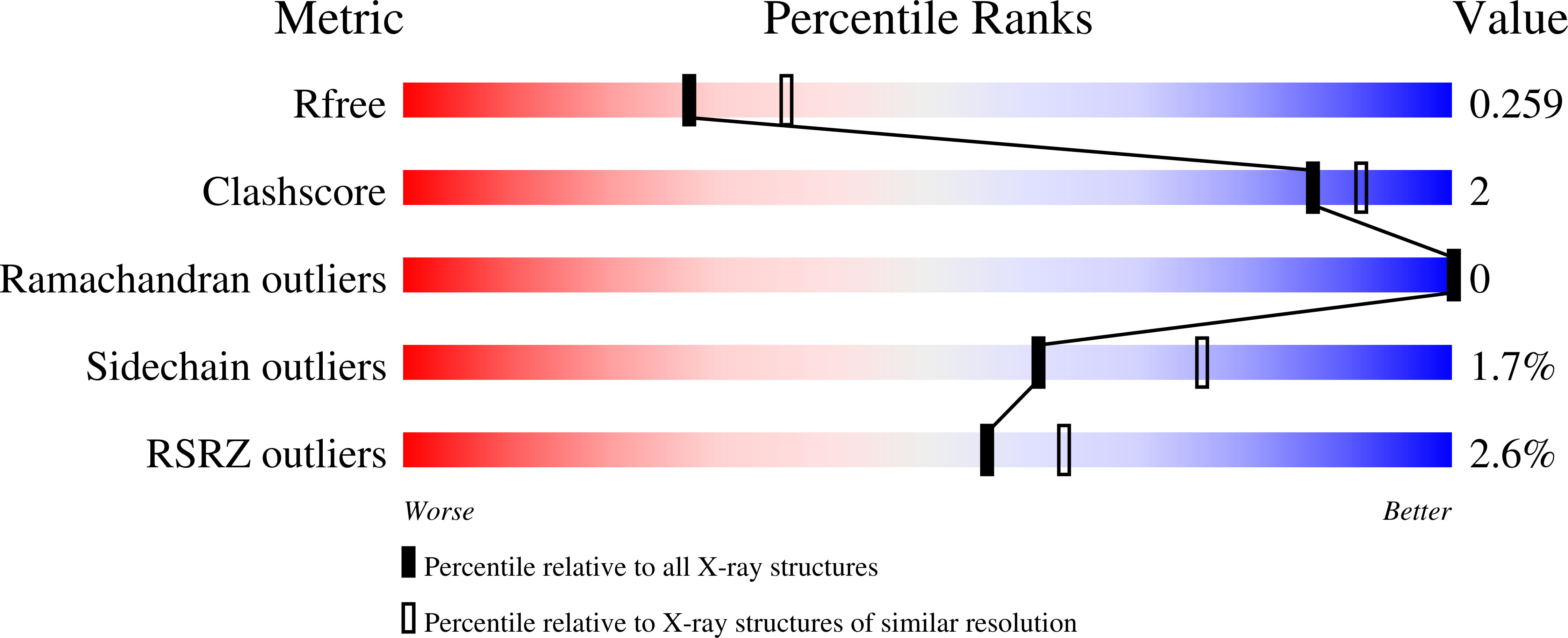
4K9R - PubMed Abstract:
Spore photoproduct lyase (SPL) repairs a covalent UV-induced thymine dimer, spore photoproduct (SP), in germinating endospores and is responsible for the strong UV resistance of endospores. SPL is a radical S-adenosyl-l-methionine (SAM) enzyme, which uses a [4Fe-4S](+) cluster to reduce SAM, generating a catalytic 5'-deoxyadenosyl radical (5'-dA(?)). This in turn abstracts a H atom from SP, generating an SP radical that undergoes β scission to form a repaired 5'-thymine and a 3'-thymine allylic radical. Recent biochemical and structural data suggest that a conserved cysteine donates a H atom to the thymine radical, resulting in a putative thiyl radical. Here we present structural and biochemical data that suggest that two conserved tyrosines are also critical in enzyme catalysis. One [Y99(Bs) in Bacillus subtilis SPL] is downstream of the cysteine, suggesting that SPL uses a novel hydrogen atom transfer (HAT) pathway with a pair of cysteine and tyrosine residues to regenerate SAM. The other tyrosine [Y97(Bs)] has a structural role to facilitate SAM binding; it may also contribute to the SAM regeneration process by interacting with the putative (?)Y99(Bs) and/or 5'-dA(?) intermediates to lower the energy barrier for the second H abstraction step. Our results indicate that SPL is the first member of the radical SAM superfamily (comprising more than 44000 members) to bear a catalytically operating HAT chain.
Organizational Affiliation:
Department of Chemistry and Chemical Biology, Indiana University-Purdue University Indianapolis, Indianapolis, IN 46202, USA.