Cofactor specificity motifs and the induced fit mechanism in class I ketol-acid reductoisomerases.
Cahn, J.K., Brinkmann-Chen, S., Spatzal, T., Wiig, J.A., Buller, A.R., Einsle, O., Hu, Y., Ribbe, M.W., Arnold, F.H.(2015) Biochem J 468: 475-484
- PubMed: 25849365
- DOI: https://doi.org/10.1042/BJ20150183
- Primary Citation of Related Structures:
4XDY, 4XDZ, 4XEH, 4XIY - PubMed Abstract:
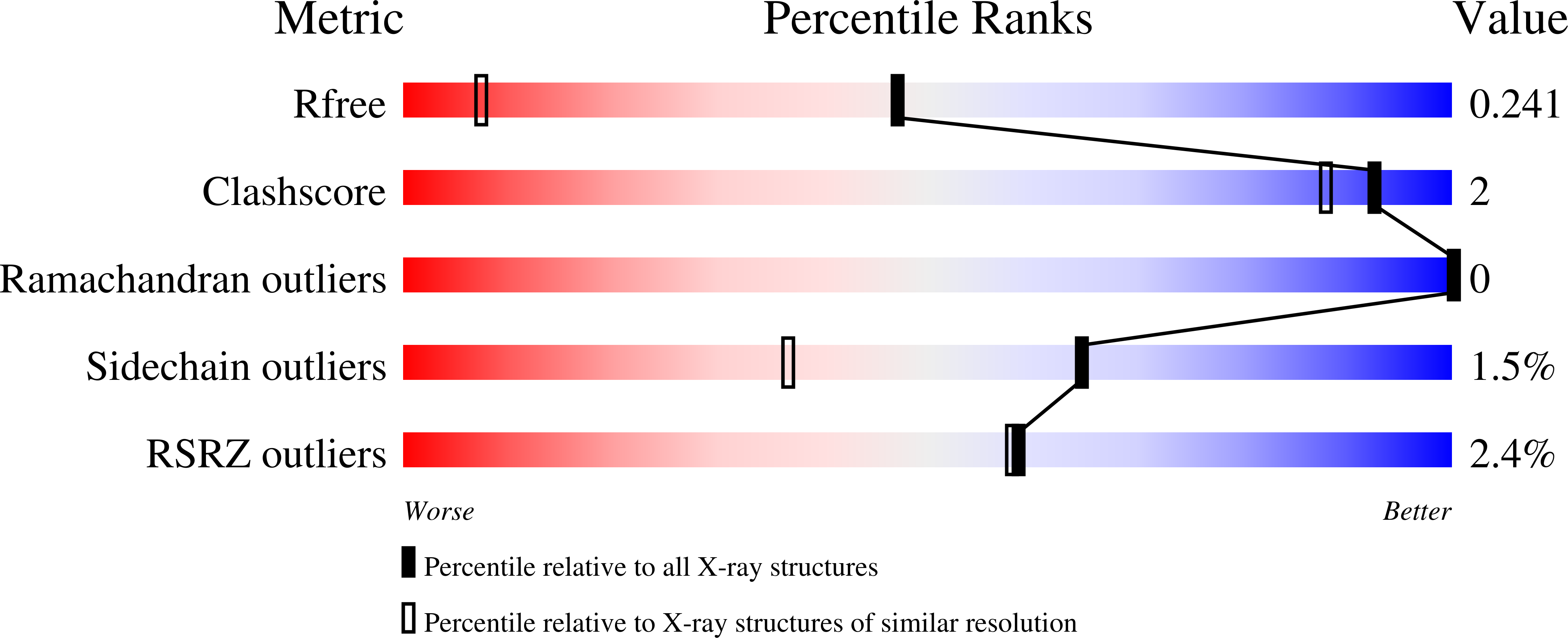
Although most sequenced members of the industrially important ketol-acid reductoisomerase (KARI) family are class I enzymes, structural studies to date have focused primarily on the class II KARIs, which arose through domain duplication. In the present study, we present five new crystal structures of class I KARIs. These include the first structure of a KARI with a six-residue ¦Â2¦ÁB (cofactor specificity determining) loop and an NADPH phosphate-binding geometry distinct from that of the seven- and 12-residue loops. We also present the first structures of naturally occurring KARIs that utilize NADH as cofactor. These results show insertions in the specificity loops that confounded previous attempts to classify them according to loop length. Lastly, we explore the conformational changes that occur in class I KARIs upon binding of cofactor and metal ions. The class I KARI structures indicate that the active sites close upon binding NAD(P)H, similar to what is observed in the class II KARIs of rice and spinach and different from the opening of the active site observed in the class II KARI of Escherichia coli. This conformational change involves a decrease in the bending of the helix that runs between the domains and a rearrangement of the nicotinamide-binding site.
Organizational Affiliation:
Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA 91125, U.S.A.