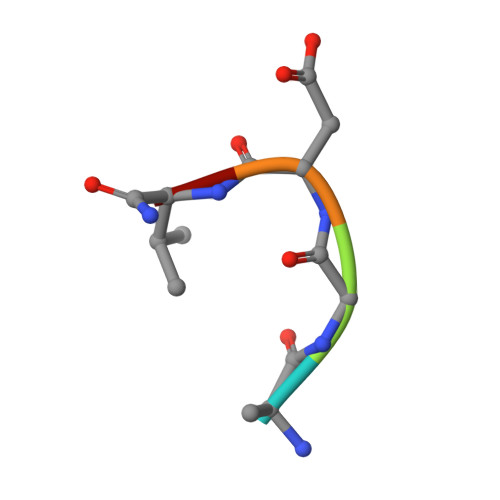
beta-Subunit Binding Is Sufficient for Ligands to Open the Integrin alpha IIb beta 3 Headpiece.
Lin, F.Y., Zhu, J., Eng, E.T., Hudson, N.E., Springer, T.A.(2016) J Biological Chem 291: 4537-4546
- PubMed: 26631735
- DOI: https://doi.org/10.1074/jbc.M115.705624
- Primary Citation of Related Structures:
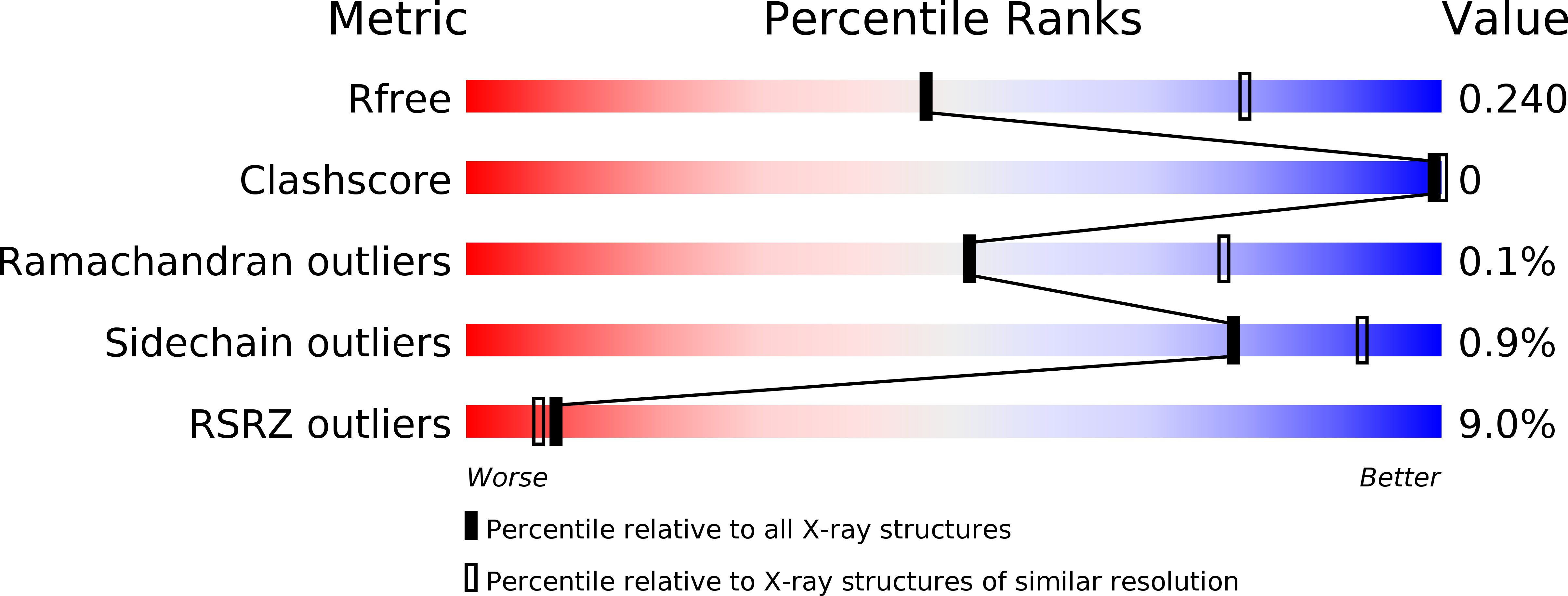
4Z7N, 4Z7O, 4Z7Q, 5HDB - PubMed Abstract:
The platelet integrin ¦ÁIIb¦Â3 binds to a KQAGDV motif at the fibrinogen ¦Ă-chain C terminus and to RGD motifs present in loops in many extracellular matrix proteins. These ligands bind in a groove between the integrin ¦Á and ¦Â-subunits; the basic Lys or Arg side chain hydrogen bonds to the ¦ÁIIb-subunit, and the acidic Asp side chain coordinates to a metal ion held by the ¦Â3-subunit. Ligand binding induces headpiece opening, with conformational change in the ¦Â-subunit. During this opening, RGD slides in the ligand-binding pocket toward ¦ÁIIb, with movement of the ¦ÂI-domain ¦Â1-¦Á1 loop toward ¦ÁIIb, enabling formation of direct, charged hydrogen bonds between the Arg side chain and ¦ÁIIb. Here we test whether ligand interactions with ¦Â3 suffice for stable ligand binding and headpiece opening. We find that the AGDV tetrapeptide from KQAGDV binds to the ¦ÁIIb¦Â3 headpiece with affinity comparable with the RGDSP peptide from fibronectin. AGDV induced complete headpiece opening in solution as shown by increase in hydrodynamic radius. Soaking of AGDV into closed ¦ÁIIb¦Â3 headpiece crystals induced intermediate states similarly to RGDSP. AGDV has very little contact with the ¦Á-subunit. Furthermore, as measured by epitope exposure, AGDV, like the fibrinogen ¦Ă C-terminal peptide and RGD, caused integrin extension on the cell surface. Thus, pushing by the ¦Â3-subunit on Asp is sufficient for headpiece opening and ligand sliding, and no pulling by the ¦ÁIIb subunit on Arg is required.
Organizational Affiliation:
From the Department of Biological Chemistry and Molecular Pharmacology, Program in Cellular and Molecular Medicine, Boston Children's Hospital, Harvard Medical School, Boston, Massachusetts 02115.