The Rational Design of Selective Benzoxazepin Inhibitors of the alpha-Isoform of Phosphoinositide 3-Kinase Culminating in the Identification of (S)-2-((2-(1-Isopropyl-1H-1,2,4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl)oxy)propanamide (GDC-0326).
Heffron, T.P., Heald, R.A., Ndubaku, C., Wei, B., Augistin, M., Do, S., Edgar, K., Eigenbrot, C., Friedman, L., Gancia, E., Jackson, P.S., Jones, G., Kolesnikov, A., Lee, L.B., Lesnick, J.D., Lewis, C., McLean, N., Mortl, M., Nonomiya, J., Pang, J., Price, S., Prior, W.W., Salphati, L., Sideris, S., Staben, S.T., Steinbacher, S., Tsui, V., Wallin, J., Sampath, D., Olivero, A.G.(2016) J Med Chem 59: 985-1002
- PubMed: 26741947
- DOI: https://doi.org/10.1021/acs.jmedchem.5b01483
- Primary Citation of Related Structures:
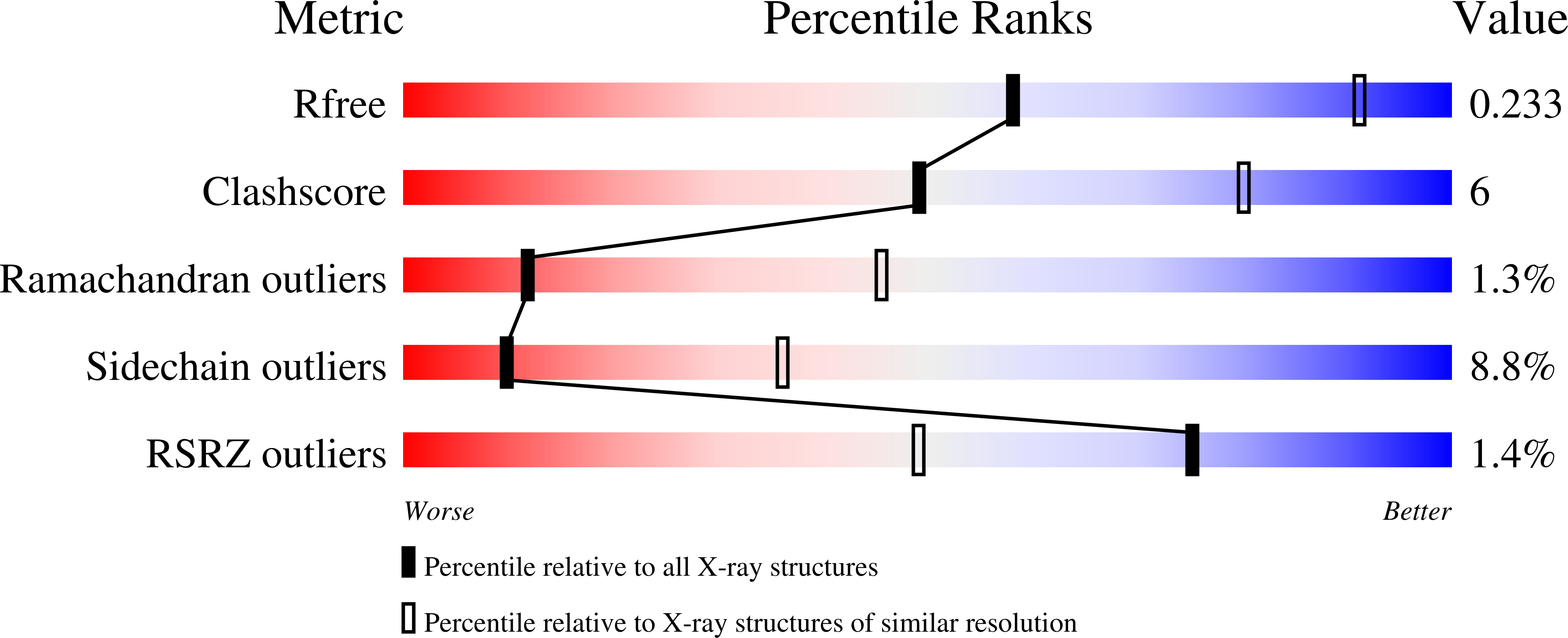
5DXH, 5DXT, 5DXU - PubMed Abstract:
Inhibitors of the class I phosphoinositide 3-kinase (PI3K) isoform PI3K¦Á have received substantial attention for their potential use in cancer therapy. Despite the particular attraction of targeting PI3K¦Á, achieving selectivity for the inhibition of this isoform has proved challenging. Herein we report the discovery of inhibitors of PI3K¦Á that have selectivity over the other class I isoforms and all other kinases tested. In GDC-0032 (3, taselisib), we previously minimized inhibition of PI3K¦Â relative to the other class I insoforms. Subsequently, we extended our efforts to identify PI3K¦Á-specific inhibitors using PI3K¦Á crystal structures to inform the design of benzoxazepin inhibitors with selectivity for PI3K¦Á through interactions with a nonconserved residue. Several molecules selective for PI3K¦Á relative to the other class I isoforms, as well as other kinases, were identified. Optimization of properties related to drug metabolism then culminated in the identification of the clinical candidate GDC-0326 (4).
Organizational Affiliation:
Genentech, Inc. , 1 DNA Way, South San Francisco, California 94080, United States.