Dynamic undocking and the quasi-bound state as tools for drug discovery.
Ruiz-Carmona, S., Schmidtke, P., Luque, F.J., Baker, L., Matassova, N., Davis, B., Roughley, S., Murray, J., Hubbard, R., Barril, X.(2017) Nat Chem 9: 201-206
- PubMed: 28221352
- DOI: https://doi.org/10.1038/nchem.2660
- Primary Citation of Related Structures:
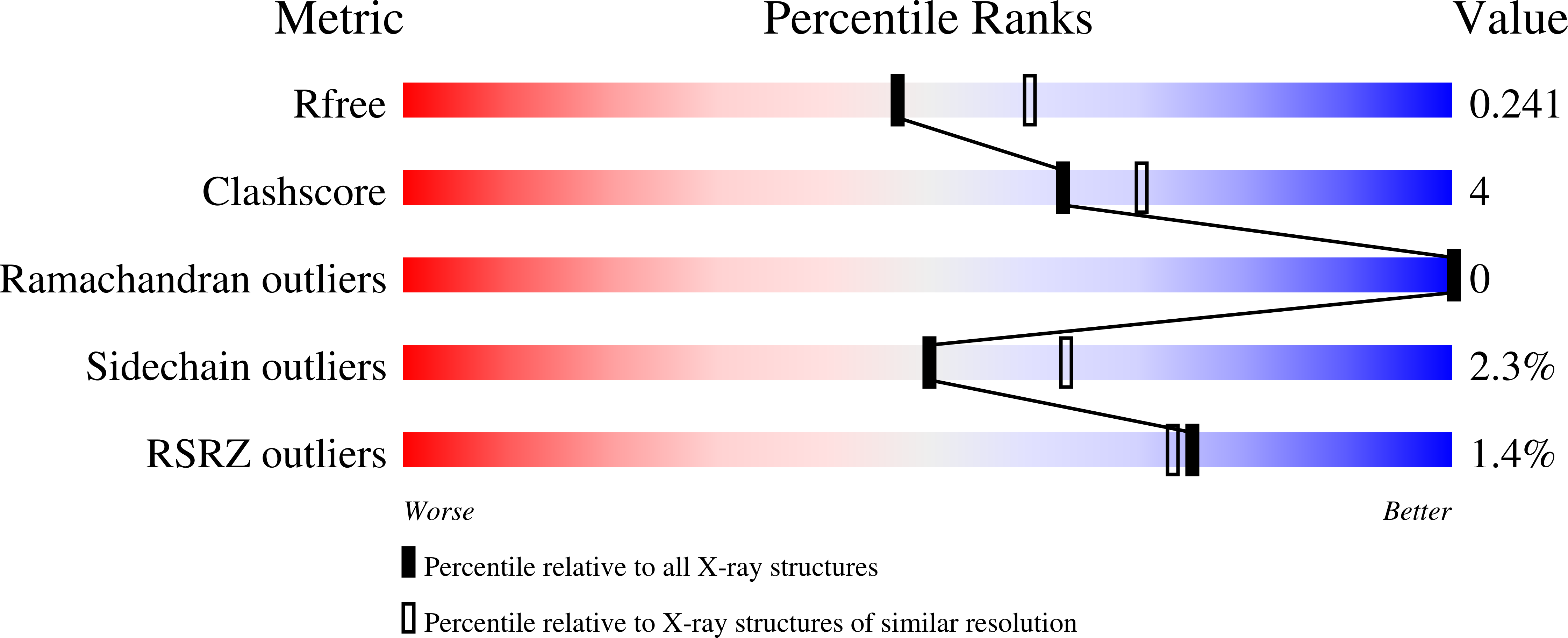
5FNC, 5FND, 5FNF - PubMed Abstract:
There is a pressing need for new technologies that improve the efficacy and efficiency of drug discovery. Structure-based methods have contributed towards this goal but they focus on predicting the binding affinity of protein-ligand complexes, which is notoriously difficult. We adopt an alternative approach that evaluates structural, rather than thermodynamic, stability. As bioactive molecules present a static binding mode, we devised dynamic undocking (DUck), a fast computational method to calculate the work necessary to reach a quasi-bound state at which the ligand has just broken the most important native contact with the receptor. This non-equilibrium property is surprisingly effective in virtual screening because true ligands form more-resilient interactions than decoys. Notably, DUck is orthogonal to docking and other 'thermodynamic' methods. We demonstrate the potential of the docking-undocking combination in a fragment screening against the molecular chaperone and oncology target Hsp90, for which we obtain novel chemotypes and a hit rate that approaches 40%.
Organizational Affiliation:
Institut de Biomedicina de la Universitat de Barcelona (IBUB) and Facultat de Farm¨¤cia, Universitat de Barcelona, Av. Joan XXIII s/n, 08028 Barcelona, Spain.