Crystal Structure Analysis of C-Phycoerythrin from Marine Cyanobacterium Phormidium Sp. A09Dm.
Kumar, V., Sonani, R.R., Sharma, M., Gupta, G.D., Madamwar, D.(2016) Photosynth Res 129: 17
- PubMed: 27068646
- DOI: https://doi.org/10.1007/s11120-016-0259-5
- Primary Citation of Related Structures:
5AQD, 5FVB - PubMed Abstract:
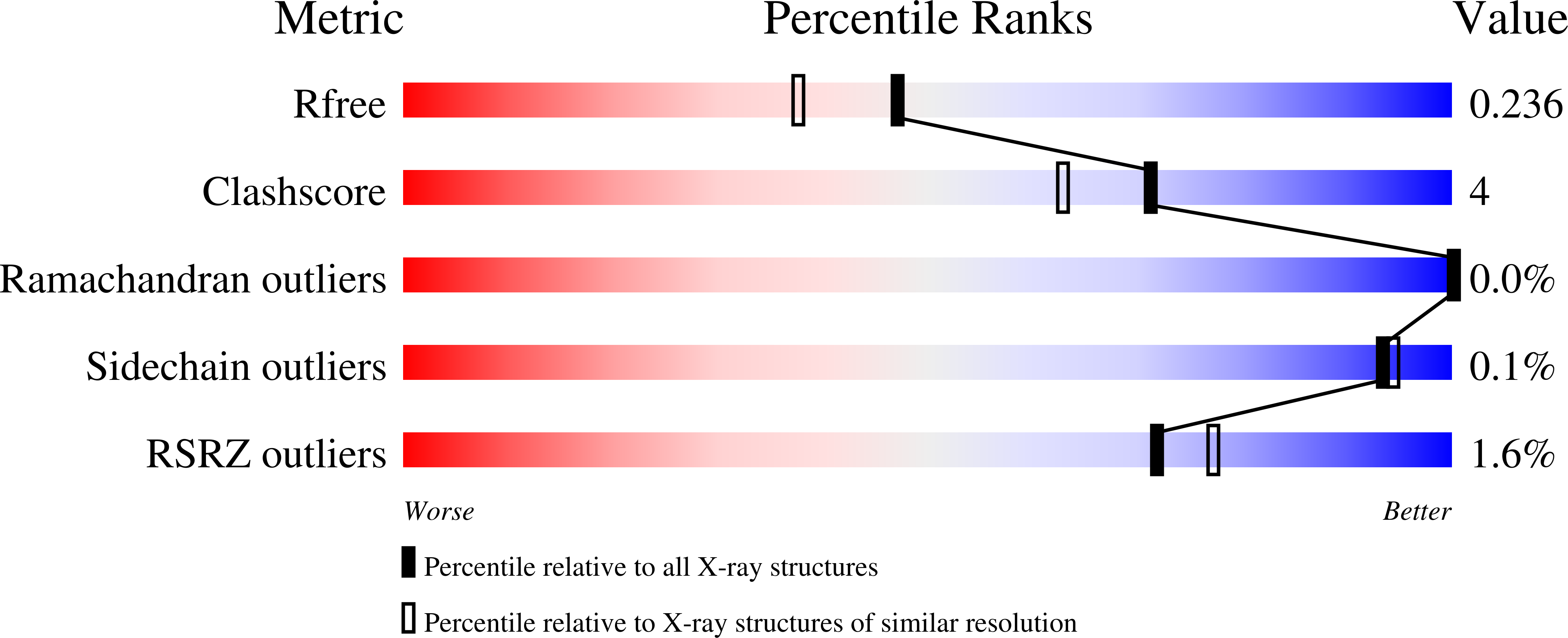
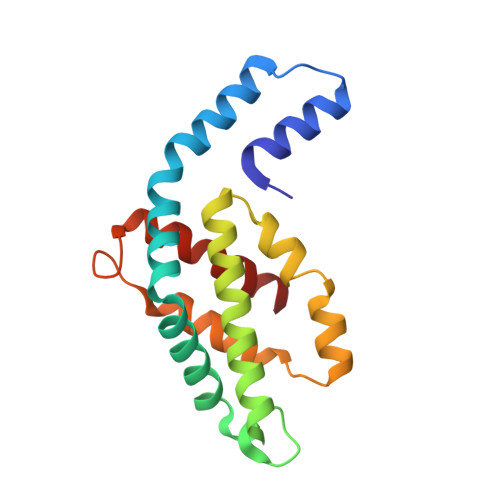
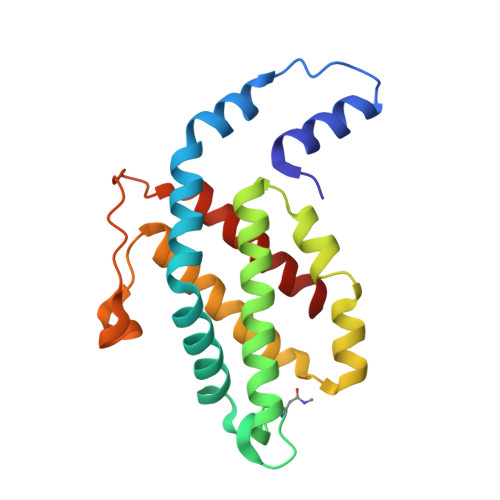
The role of unique sequence features of C-phycoerythrin, isolated from Phormidium sp. A09DM, has been investigated by crystallographic studies. Two conserved indels (i.e. inserts or deletions) are found in the ¦Â-subunit of Phormidium phycoerythrin that are distinctive characteristics of large number of cyanobacterial sequences. The identified signatures are a two-residue deletion from position 21 and a nine-residue insertion at position 146. Crystals of Phormidium phycoerythrin were obtained at pH values of 5 and 8.5, and structures have been resolved to high precision at 1.95 and 2.1?? resolution, respectively. In both the structures, heterodimers of ¦Á- and ¦Â- subunits assemble as hexamers. The 7-residue insertion at position 146 significantly reduces solvent exposure of ¦Đ-conjugated A-C rings of a phycoerythrobilin (PEB) chromophore, and can influence energy absorption and energy transfer characteristics. The structural analyses (with 12-fold redundancy) suggest that protein micro-environment alone dictates the conformation of bound chromophores. The low- and high-energy absorbing chromophores are identified based on A-B ring coplanarity. The spatial distribution of these is found to be similar to that observed in R-phycoerythrin, suggesting the direction of energy transfer from outer-surface of hexamer to inner-hollow cavity in the Phormidium protein. The crystal structures also reveal that a commonly observed Hydrogen-bonding network in phycobiliproteins, involving chromophore bound to ¦Á-subunit and amino acid at position 73 of ¦Â-subunit, may not be essential for structural and functional integrity of C-phycoerythrin orthologs. In solution, the protein displays slight red shift and decrease in fluorescence emission at acidic pH. The mechanism for which may be static and correlates with the proximity of +ve electric field of Arg148 to the C-ring of a PEB chromophore.
Organizational Affiliation:
Protein Crystallography Section, Solid State Physics Division, Bhabha Atomic Research Centre, Mumbai, 400085, India. vinay@barc.gov.in.