Activity-based probes for functional interrogation of retaining beta-glucuronidases.
Wu, L., Jiang, J., Jin, Y., Kallemeijn, W.W., Kuo, C.L., Artola, M., Dai, W., van Elk, C., van Eijk, M., van der Marel, G.A., Codee, J.D.C., Florea, B.I., Aerts, J.M.F.G., Overkleeft, H.S., Davies, G.J.(2017) Nat Chem Biol 13: 867-873
- PubMed: 28581485
- DOI: https://doi.org/10.1038/nchembio.2395
- Primary Citation of Related Structures:
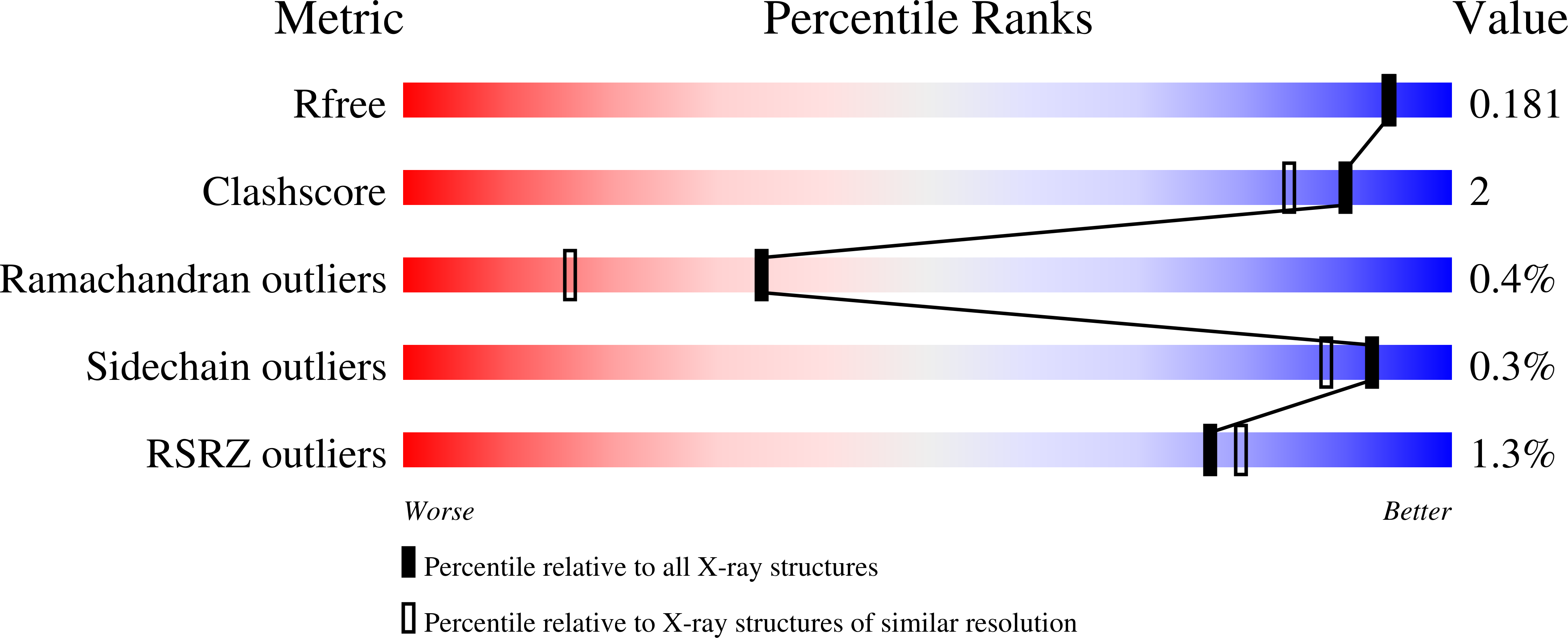
5G0Q, 5L77, 5L9Y, 5L9Z, 5LA4, 5LA7 - PubMed Abstract:
Humans express at least two distinct ¦Â-glucuronidase enzymes that are involved in disease: exo-acting ¦Â-glucuronidase (GUSB), whose deficiency gives rise to mucopolysaccharidosis type VII, and endo-acting heparanase (HPSE), whose overexpression is implicated in inflammation and cancers. The medical importance of these enzymes necessitates reliable methods to assay their activities in tissues. Herein, we present a set of ¦Â-glucuronidase-specific activity-based probes (ABPs) that allow rapid and quantitative visualization of GUSB and HPSE in biological samples, providing a powerful tool for dissecting their activities in normal and disease states. Unexpectedly, we find that the supposedly inactive HPSE proenzyme proHPSE is also labeled by our ABPs, leading to surprising insights regarding structural relationships between proHPSE, mature HPSE, and their bacterial homologs. Our results demonstrate the application of ¦Â-glucuronidase ABPs in tracking pathologically relevant enzymes and provide a case study of how ABP-driven approaches can lead to discovery of unanticipated structural and biochemical functionality.
Organizational Affiliation:
York Structural Biology Laboratory, Department of Chemistry, University of York, Heslington, York, UK.