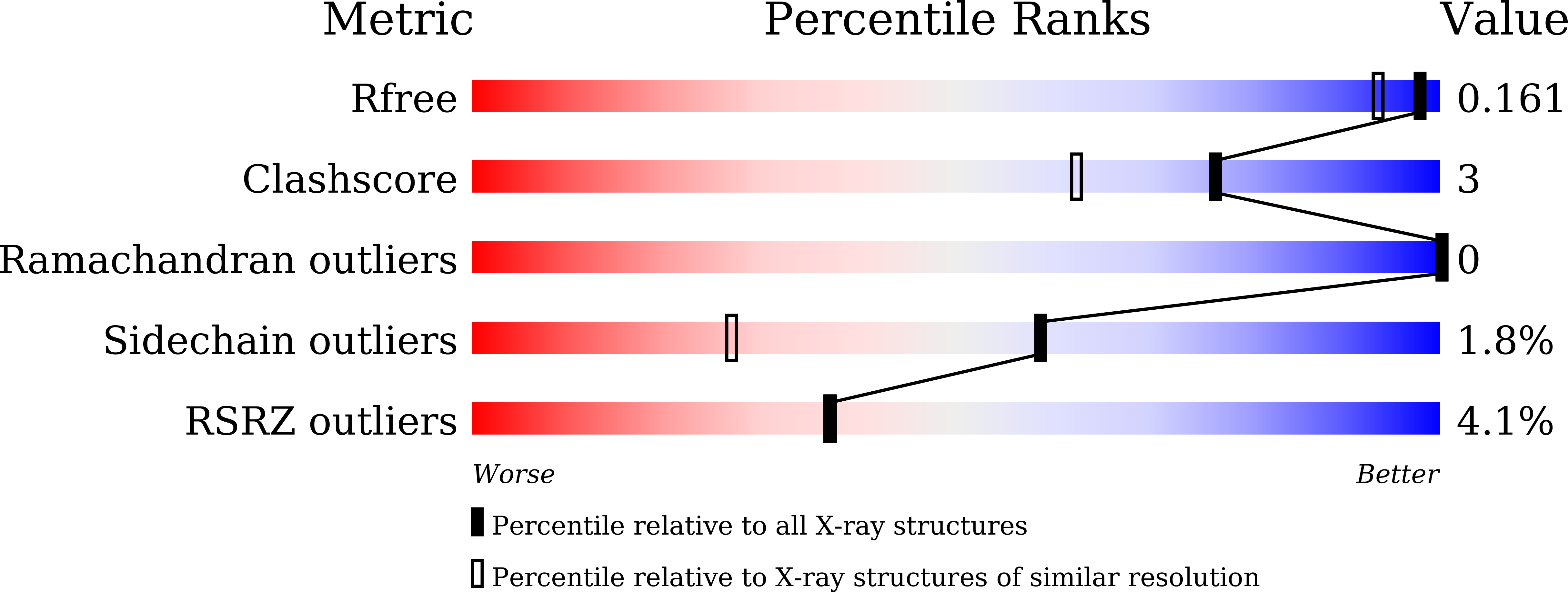
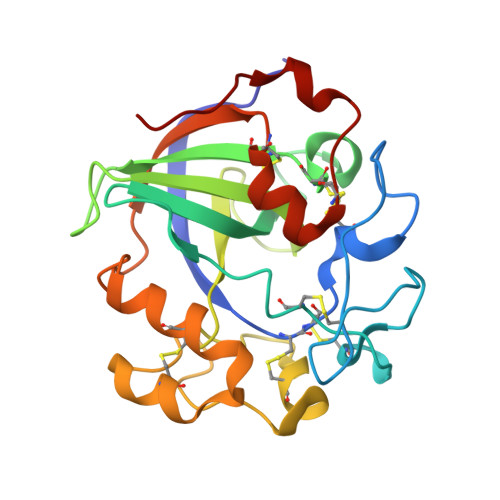
Characterization and crystal structure of a thermostable glycoside hydrolase family 45 1,4-beta-endoglucanase from Thielavia terrestris
Gao, J., Huang, J.W., Li, Q., Liu, W.D., Ko, T.P., Zheng, Y.Y., Xiao, X., Kuo, C.J., Chen, C.C., Guo, R.T.(2017) Enzyme Microb Technol 99: 32-37
- PubMed: 28193329
- DOI: https://doi.org/10.1016/j.enzmictec.2017.01.005
- Primary Citation of Related Structures:
5GLX, 5GLY, 5GM9 - PubMed Abstract:
1,4-¦Â-Endoglucanase is one of the most important biocatalysts in modern industries. Here, a glycoside hydrolase (GH) family 45 endoglucanase from thermophilic fungus Theilavia terrestris (TtCel45A) was expressed in Pichia pastoris. The recombinant protein shows optimal activity at 60¡ãC, pH 4-5. The enzyme exhibits extraordinary thermostability that more than 80% activity was detected after heating at 80¡ãC for 2.5h. The high resolution crystal structures of apo-form enzyme and that in complex with cellobiose and cellotetraose were solved to 1.36-1.58?. The protein folds into two overall regions: one is a six-stranded ¦Â-barrel, and the other one consists of several extended loops. Between the two regions lies the substrate-binding channel, which is an open cleft spanning across the protein surface. A continuous substrate-binding cleft from subsite -4 to +3 were clearly identified in the complex structures. Notably, the flexible V-VI loop ( 113 Gly- 114 Gly- 115 Asp- 116 Leu- 117 Gly- 118 Ser) is found to open in the presence of -1 sugar, with D115 and L116 swung away to yield a space to accommodate the catalytic acid D122 and the 2,5 B boat conformation of -1 sugar during transition state. Collectively, we characterized the enzyme properties of P. pastoris-expressed TtCel45A and solved high-resolution crystal structures of the enzyme. These results are of great interests in industrial applications and provide new insights into the fundamental understanding of enzyme catalytic mechanism of GH45 endoglucanases.
Organizational Affiliation:
Industrial Enzymes National Engineering Laboratory, Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, Tianjin, 300308, China.