NMR Fragment Screening Hit Induces Plasticity of BRD7/9 Bromodomains
Wang, N., Li, F., Bao, H., Li, J., Wu, J., Ruan, K.(2016) Chembiochem 17: 1456-1463
- PubMed: 27194508
- DOI: https://doi.org/10.1002/cbic.201600184
- Primary Citation of Related Structures:
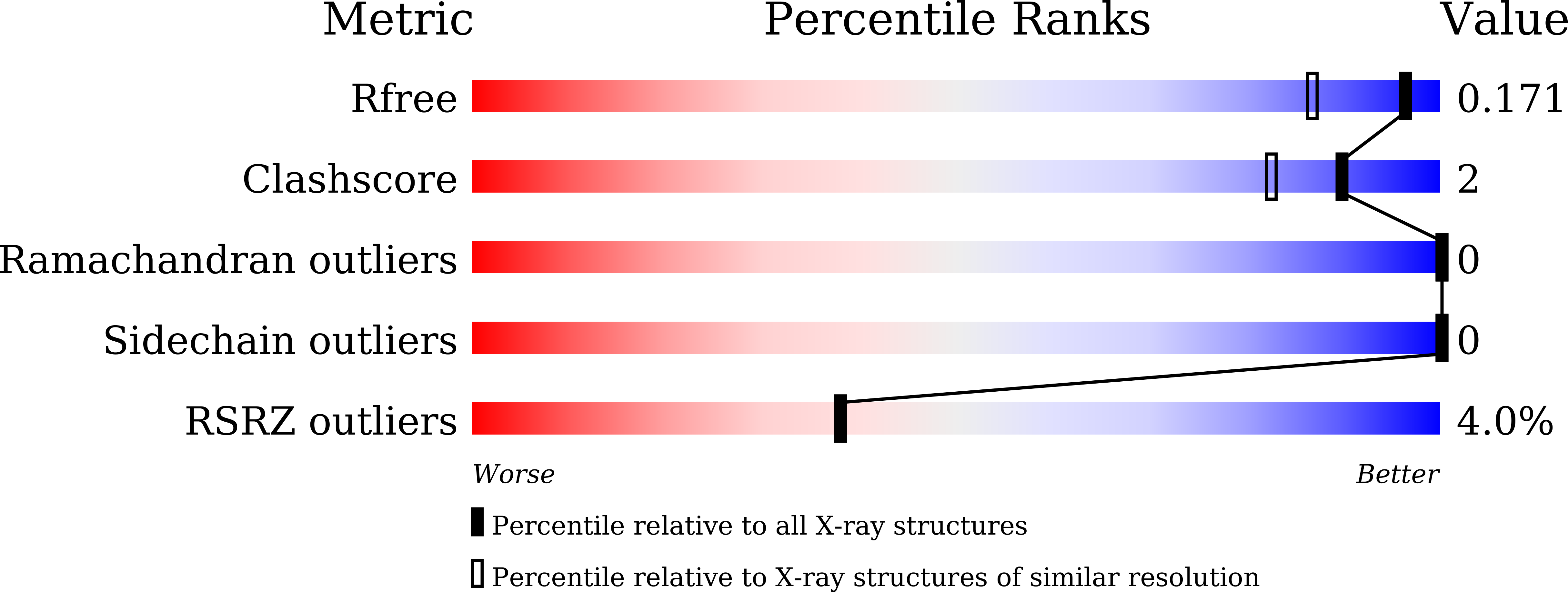
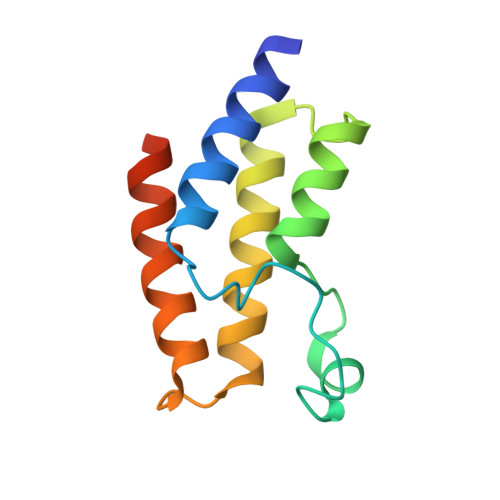
5JI8 - PubMed Abstract:
The complex biology associated with inhibition of bromodomain and extra-terminal (BET) domains by chemical probes has attracted increasing attention, and there is a need to identify non-BET bromodomain (BD) inhibitors. Several potent inhibitors of the BRD9 BD have recently been discovered, with anticancer and anti-inflammation activity. However, its paralogue, BRD7 BD, remains unexploited. Here, we identified new chemotypes targeting BRD7 BD by using NMR fragment-based screening. BRD7/9 BDs exhibit similar patterns of chemical-shift perturbation upon the titration of hit compound 1. The crystal structure revealed that 1 repels the Y222 group of BRD9 BD in a similar way to that for butyryllysine, but not acetyllysine and known inhibitors. Hit?1 induced less rearrangement of residue F161 of BRD9 BD than acetyllysine, butyryllysine, and crotonyllysine. Our study provides structural insight into a new generation of butyryllysine mimics for probing the function of BRD7/9 BD.
Organizational Affiliation:
Hefei National Laboratory for Physical Science at the Microscale, School of Life Sciences, University of Science and Technology of China, Hefei, Anhui, 230027, China.