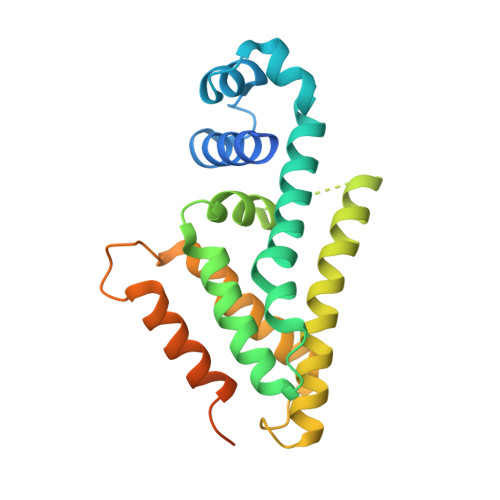
The AibR-isovaleryl coenzyme A regulator and its DNA binding site - a model for the regulation of alternative de novo isovaleryl coenzyme A biosynthesis in Myxococcus xanthus.
Bock, T., Volz, C., Hering, V., Scrima, A., Muller, R., Blankenfeldt, W.(2017) Nucleic Acids Res 45: 2166-2178
- PubMed: 27940564
- DOI: https://doi.org/10.1093/nar/gkw1238
- Primary Citation of Related Structures:
5K7F, 5K7H - PubMed Abstract:
Isovaleryl coenzyme A (IV-CoA) is an important building block of iso-fatty acids. In myxobacteria, IV-CoA is essential for the formation of signaling molecules involved in fruiting body formation. Leucine degradation is the common source of IV-CoA, but a second, de novo biosynthetic route to IV-CoA termed AIB (alternative IV-CoA biosynthesis) was recently discovered in M. xanthus. The AIB-operon contains the TetR-like transcriptional regulator AibR, which we characterize in this study. We demonstrate that IV-CoA binds AibR with micromolar affinity and show by gelshift experiments that AibR interacts with the promoter region of the AIB-operon once IV-CoA is present. We identify an 18-bp near-perfect palindromic repeat as containing the AibR operator and provide evidence that AibR also controls an additional genomic locus coding for a putative acetyl-CoA acetyltransferase. To elucidate atomic details, we determined crystal structures of AibR in the apo, the IV-CoA- and the IV-CoA-DNA-bound state to 1.7 ?, 2.35 ? and 2.92 ?, respectively. IV-CoA induces partial unfolding of an ¦Á-helix, which allows sequence-specific interactions between AibR and its operator. This study provides insights into AibR-mediated regulation and shows that AibR functions in an unusual TetR-like manner by blocking transcription not in the ligand-free but in the effector-bound state.
Organizational Affiliation:
Structure and Function of Proteins, Helmholtz Centre for Infection Research, Inhoffenstr. 7, 38124 Braunschweig, Germany.