Shuffling Active Site Substate Populations Affects Catalytic Activity: The Case of Glucose Oxidase.
Petrovic, D., Frank, D., Kamerlin, S.C.L., Hoffmann, K., Strodel, B.(2017) ACS Catal 7: 6188-6197
- PubMed: 29291138
- DOI: https://doi.org/10.1021/acscatal.7b01575
- Primary Citation of Related Structures:
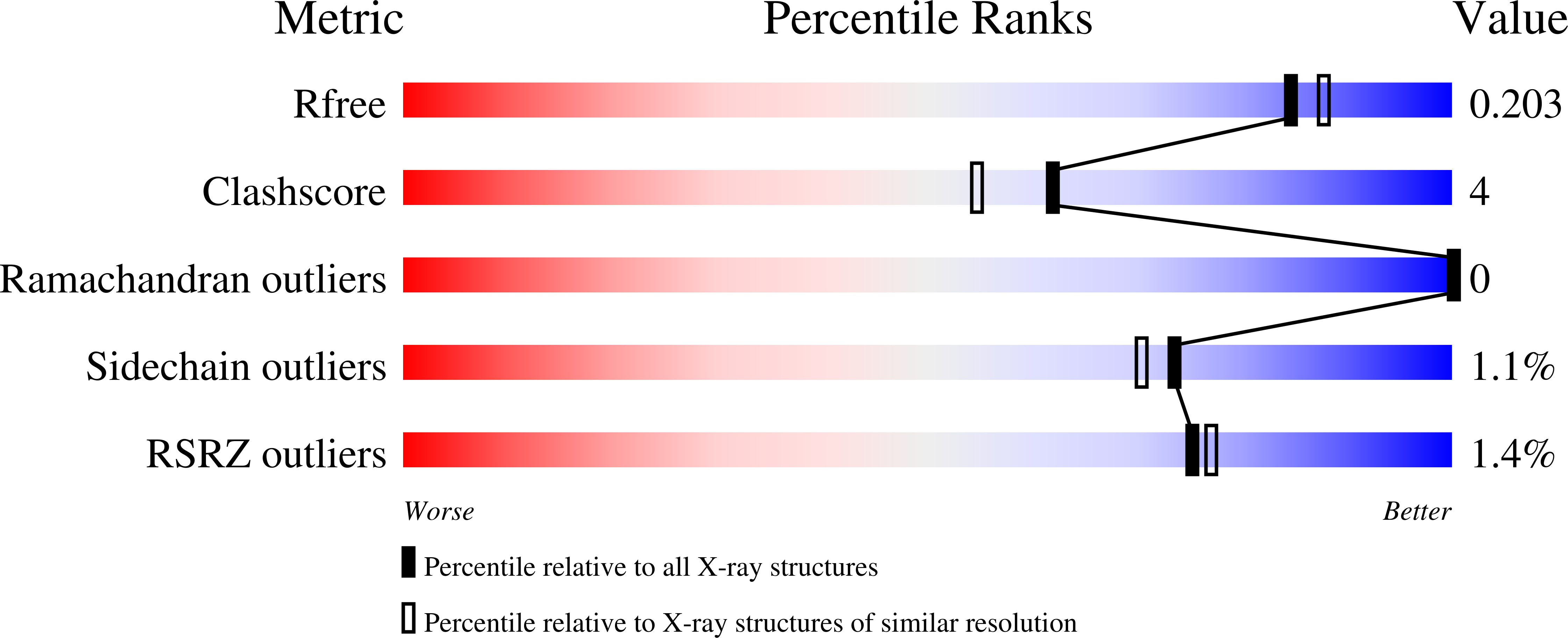
5NIT, 5NIW - PubMed Abstract:
Glucose oxidase has wide applications in the pharmaceutical, chemical, and food industries. Many recent studies have enhanced key properties of this enzyme using directed evolution, yet without being able to reveal why these mutations are actually beneficial. This work presents a synergistic combination of experimental and computational methods, indicating how mutations, even when distant from the active site, positively affect glucose oxidase catalysis. We have determined the crystal structures of glucose oxidase mutants containing molecular oxygen in the active site. The catalytically important His516 residue has been previously shown to be flexible in the wild-type enzyme. The molecular dynamics simulations performed in this work allow us to quantify this floppiness, revealing that His516 exists in two states: catalytic and noncatalytic. The relative populations of these two substates are almost identical in the wild-type enzyme, with His516 readily shuffling between them. In the glucose oxidase mutants, on the other hand, the mutations enrich the catalytic His516 conformation and reduce the flexibility of this residue, leading to an enhancement in their catalytic efficiency. This study stresses the benefit of active site preorganization with respect to enzyme conversion rates by reducing molecular reorientation needs. We further suggest that the computational approach based on Hamiltonian replica exchange molecular dynamics, used in this study, may be a general approach to screening in silico for improved enzyme variants involving flexible catalytic residues.
Organizational Affiliation:
Institute of Complex Systems: Structural Biochemistry, Forschungszentrum J¨¹lich, 52425 J¨¹lich, Germany.