Second-generation CK2 alpha inhibitors targeting the alpha D pocket.
Iegre, J., Brear, P., De Fusco, C., Yoshida, M., Mitchell, S.L., Rossmann, M., Carro, L., Sore, H.F., Hyvonen, M., Spring, D.R.(2018) Chem Sci 9: 3041-3049
- PubMed: 29732088
- DOI: https://doi.org/10.1039/c7sc05122k
- Primary Citation of Related Structures:
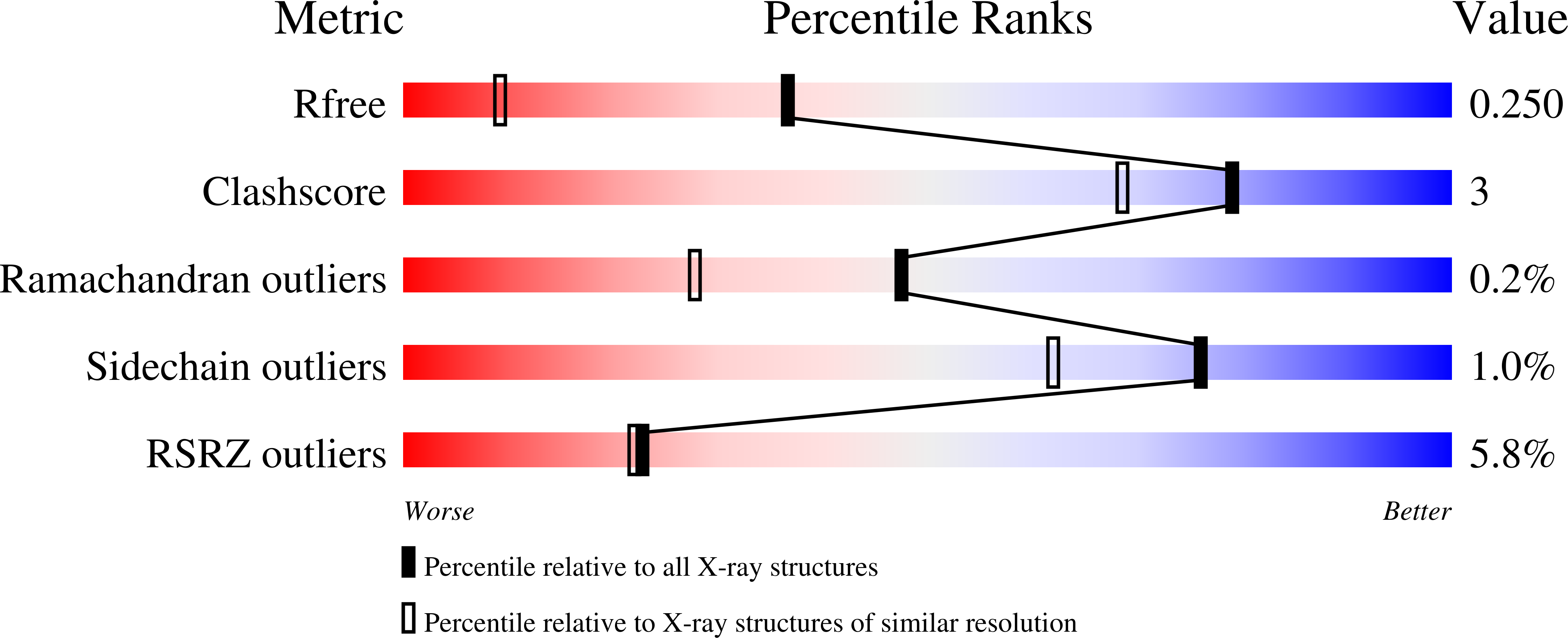
5OQU, 5ORH, 5ORJ, 5ORK, 5OS7, 5OS8, 5OSL, 5OSP, 5OSR, 5OSU, 5OSZ, 5OT5, 5OT6, 5OTD, 5OTH, 5OTI, 5OTL, 5OTO, 5OTP, 5OTQ, 5OTR, 5OTS, 5OTY, 5OTZ, 5OUE, 5OUL, 5OUM, 5OUU, 5OYF, 6EHK, 6EHU, 6EII - PubMed Abstract:
CK2 is a critical cell cycle regulator that also promotes various anti-apoptotic mechanisms. Development of ATP-non-competitive inhibitors of CK2 is a very attractive strategy considering that the ATP binding site is highly conserved among other kinases. We have previously utilised a pocket outside the active site to develop a novel CK2 inhibitor, CAM4066 . Whilst CAM4066 bound to this new pocket it was also interacting with the ATP site: herein, we describe an example of a CK2¦Á inhibitor that binds completely outside the active site. This second generation ¦ÁD-site binding inhibitor, compound CAM4712 (IC 50 = 7 ¦ÌM, GI 50 = 10.0 ¡À 3.6 ¦ÌM), has numerous advantages over the previously reported CAM4066 , including a reduction in the number of rotatable bonds, the absence of amide groups susceptible to the action of proteases and improved cellular permeability. Unlike with CAM4066 , there was no need to facilitate cellular uptake by making a prodrug. Moreover, CAM4712 displayed no drop off between its ability to inhibit the kinase in vitro (IC 50 ) and the ability to inhibit cell proliferation (GI 50 ).
Organizational Affiliation:
Department of Chemistry , University of Cambridge , CB2 1EW , Cambridge , UK . Email: spring@ch.cam.ac.uk.