Time-Dependent Extension from an 8-Oxoguanine Lesion by Human DNA Polymerase Beta.
Reed, A.J., Suo, Z.(2017) J Am Chem Soc 139: 9684-9690
- PubMed: 28682600
- DOI: https://doi.org/10.1021/jacs.7b05048
- Primary Citation of Related Structures:
5VRW, 5VRX, 5VRY, 5VRZ, 5VS0, 5VS1, 5VS2, 5VS3, 5VS4 - PubMed Abstract:
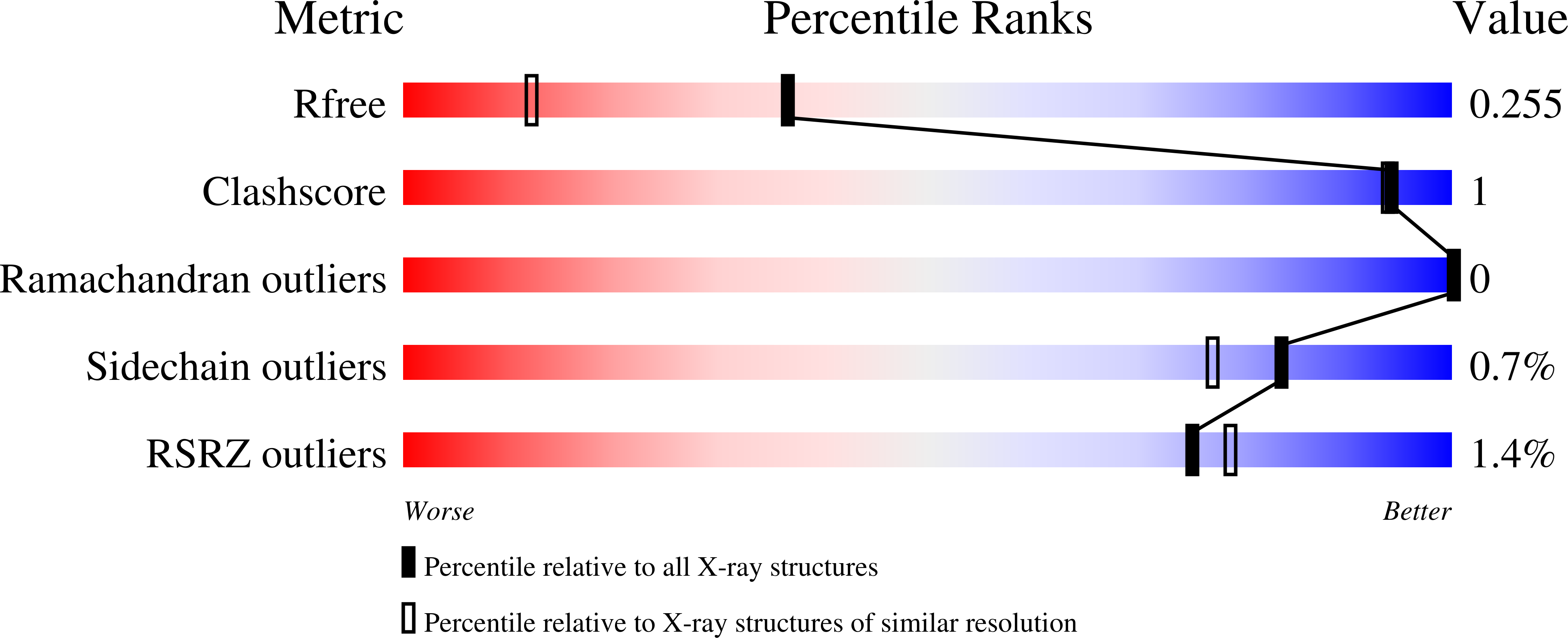
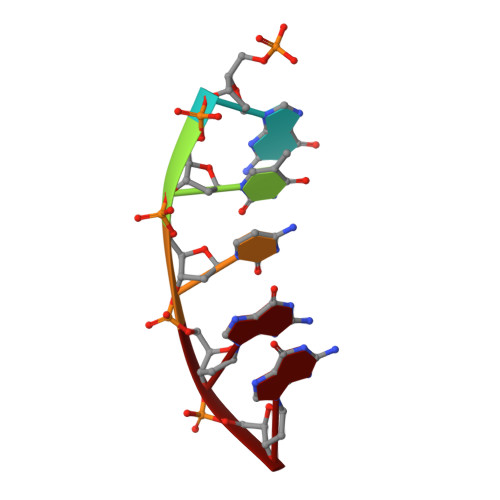
The oxidative DNA lesion 7,8-dihydro-2'-deoxyguanine (8-oxoG) often occurs in double-stranded DNA and poses a threat to genomic integrity due to the ability of 8-oxoG to form stable Watson-Crick base pairs with deoxycytidine (8-oxoG:dC) and Hoogsteen base pairs with deoxyadenosine (8-oxoG:dA). In humans, short-patch base excision repair of 8-oxoG:dA base pairs requires human DNA polymerase ¦Â (hPol¦Â) to bypass 8-oxoG. Previously, we have shown hPol¦Â-catalyzed 8-oxoG bypass to exhibit low fidelity and identified a unique stacking interaction between the newly incorporated nucleotide (dCMP or dAMP) and the templating 8-oxoG. The effect of this stacking on the ability of hPol¦Â to extend from 8-oxoG during long-patch base excision repair was unknown. Here we report pre-steady-state kinetics and time-dependent crystal structures to demonstrate that extension from both 8-oxoG:dC and 8-oxoG:dA base pairs is 18- to 580-fold less efficient compared to 8-oxoG bypass and that extension from 8-oxoG:dC over 8-oxoG:dA is favored by 15-fold. The overall decrease in efficiency of extension relative to 8-oxoG bypass is due to an alternative nucleotide binding conformation in the precatalytic ternary structures (hPol¦Âˇ¤DNAˇ¤dNTP) for both extension contexts, wherein the incoming nucleotide is bound in either the canonical Watson-Crick base pair or a nonplanar base pair. In addition, the decreased stability of the ternary complex of 8-oxoG:dA extension results in further loss of efficiency when compared to 8-oxoG:dC extension. Therefore, we hypothesize that the inefficient extension from 8-oxoG:dA serves as a newly discovered fidelity checkpoint during base excision repair.
Organizational Affiliation:
Department of Chemistry and Biochemistry, The Ohio State Biochemistry Program, The Ohio State University , Columbus, Ohio 43210, United States.