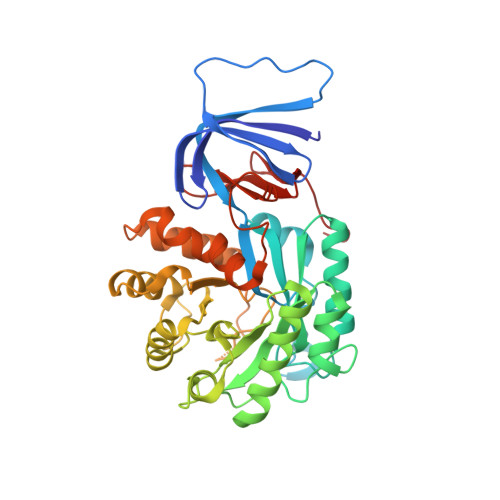
Crystal structure and functional characterization of an isoaspartyl dipeptidase (CpsIadA) from Colwellia psychrerythraea strain 34H.
Park, S.H., Lee, C.W., Lee, S.G., Shin, S.C., Kim, H.J., Park, H., Lee, J.H.(2017) PLoS One 12: e0181705-e0181705
- PubMed: 28723955
- DOI: https://doi.org/10.1371/journal.pone.0181705
- Primary Citation of Related Structures:
5XGW, 5XGX - PubMed Abstract:
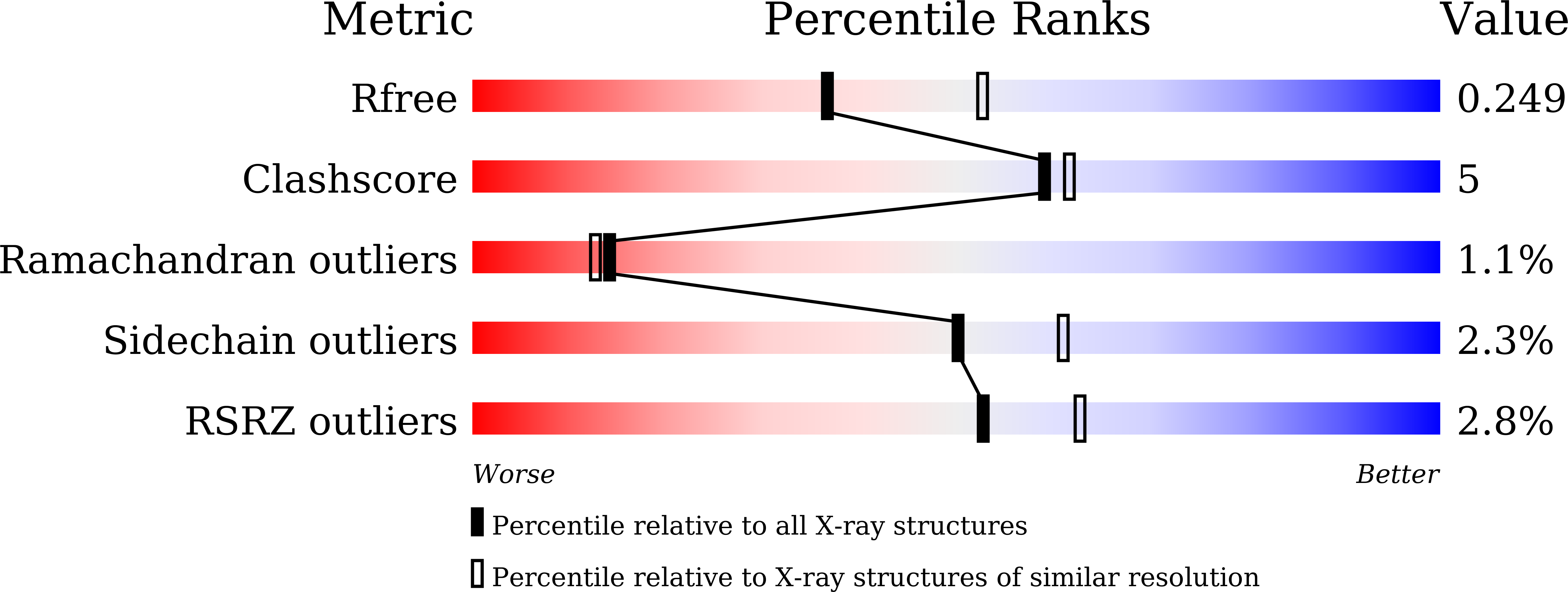
Isoaspartyl dipeptidase (IadA) is an enzyme that catalyzes the hydrolysis of an isoaspartyl dipeptide-like moiety, which can be inappropriately formed in proteins, between the ¦Â-carboxyl group side chain of Asp and the amino group of the following amino acid. Here, we have determined the structures of an isoaspartyl dipeptidase (CpsIadA) from Colwellia psychrerythraea, both ligand-free and that complexed with ¦Â-isoaspartyl lysine, at 1.85-? and 2.33-? resolution, respectively. In both structures, CpsIadA formed an octamer with two Zn ions in the active site. A structural comparison with Escherichia coli isoaspartyl dipeptidase (EcoIadA) revealed a major difference in the structure of the active site. For metal ion coordination, CpsIadA has a Glu166 residue in the active site, whereas EcoIadA has a post-translationally carbamylated-lysine 162 residue. Site-directed mutagenesis studies confirmed that the Glu166 residue is critical for CpsIadA enzymatic activity. This residue substitution from lysine to glutamate induces the protrusion of the ¦Â12-¦Á8 loop into the active site to compensate for the loss of length of the side chain. In addition, the ¦Á3-¦Â9 loop of CpsIadA adopts a different conformation compared to EcoIadA, which induces a change in the structure of the substrate-binding pocket. Despite CpsIadA having a different active-site residue composition and substrate-binding pocket, there is only a slight difference in CpsIadA substrate specificity compared with EcoIadA. Comparative sequence analysis classified IadA-containing bacteria and archaea into two groups based on the active-site residue composition, with Type I IadAs having a glutamate residue and Type II IadAs having a carbamylated-lysine residue. CpsIadA has maximal activity at pH 8-8.5 and 45¡ãC, and was completely inactivated at 60¡ãC. Despite being isolated from a psychrophilic bacteria, CpsIadA is thermostable probably owing to its octameric structure. This is the first conclusive description of the structure and properties of a Type I IadA.
Organizational Affiliation:
Unit of Polar Genomics, Korea Polar Research Institute, Incheon, Republic of Korea.