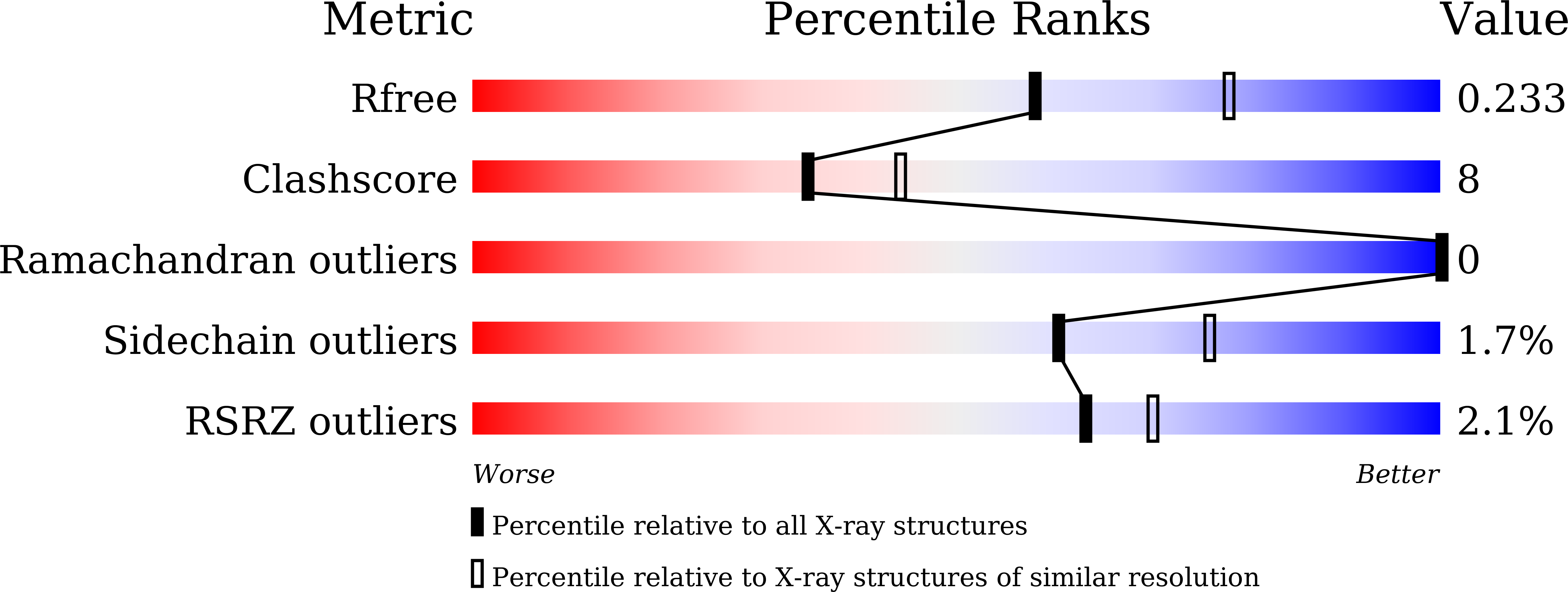
Crystallographic Structures of IlvNˇ¤Val/Ile Complexes: Conformational Selectivity for Feedback Inhibition of Aceto Hydroxy Acid Synthases.
Bansal, A., Karanth, N.M., Demeler, B., Schindelin, H., Sarma, S.P.(2019) Biochemistry 58: 1992-2008
- PubMed: 30887800
- DOI: https://doi.org/10.1021/acs.biochem.9b00050
- Primary Citation of Related Structures:
5YPP, 5YPW, 5YPY, 5YUM - PubMed Abstract:
Conformational factors that predicate selectivity for valine or isoleucine binding to IlvN leading to the regulation of aceto hydroxy acid synthase I (AHAS I) of Escherichia coli have been determined for the first time from high-resolution (1.9-2.43 ?) crystal structures of IlvNˇ¤Val and IlvNˇ¤Ile complexes. The valine and isoleucine ligand binding pockets are located at the dimer interface. In the IlvNˇ¤Ile complex, among residues in the binding pocket, the side chain of Cys 43 is 2-fold disordered (¦Ö 1 angles of gauche - and trans). Only one conformation can be observed for the identical residue in the IlvNˇ¤Val complexes. In a reversal, the side chain of His 53 , located at the surface of the protein, exhibits two conformations in the IlvNˇ¤Val complex. The concerted conformational switch in the side chains of Cys 43 and His 53 may play an important role in the regulation of the AHAS I holoenzyme activity. A significant result is the establishment of the subunit composition in the AHAS I holoenzyme by analytical ultracentrifugation. Solution nuclear magnetic resonance and analytical ultracentrifugation experiments have also provided important insights into the hydrodynamic properties of IlvN in the ligand-free and -bound states. The structural and biophysical data unequivocally establish the molecular basis for differential binding of the ligands to IlvN and a rationale for the resistance of IlvM to feedback inhibition by the branched-chain amino acids.
Organizational Affiliation:
Molecular Biophysics Unit , Indian Institute of Science , Bangalore , Karnataka 560012 , India.