Active-Site Conformational Fluctuations Promote the Enzymatic Activity of NDM-1.
Zhang, H., Ma, G., Zhu, Y., Zeng, L., Ahmad, A., Wang, C., Pang, B., Fang, H., Zhao, L., Hao, Q.(2018) Antimicrob Agents Chemother 62
- PubMed: 30150473
- DOI: https://doi.org/10.1128/AAC.01579-18
- Primary Citation of Related Structures:
5ZGE, 5ZGF, 5ZGI, 5ZGP, 5ZGQ, 5ZGR, 5ZGT, 5ZGU, 5ZGV, 5ZGW, 5ZGX, 5ZGY, 5ZGZ, 5ZH1 - PubMed Abstract:
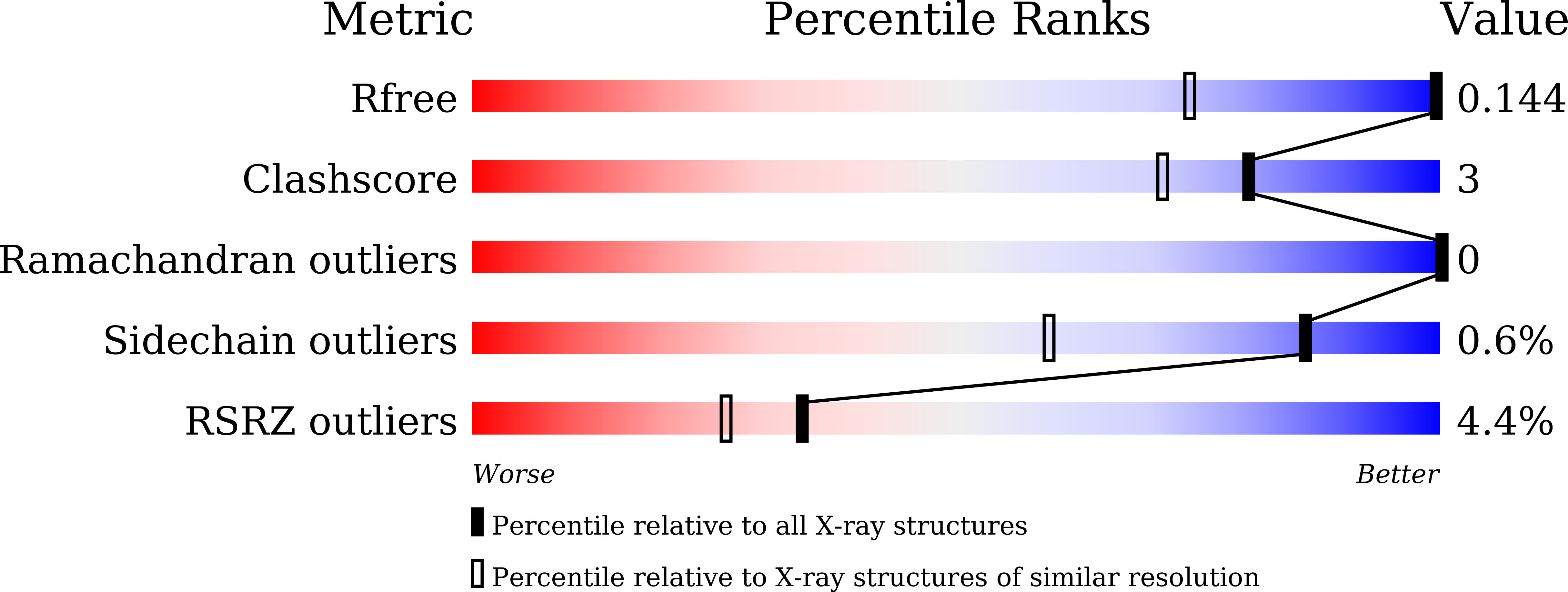
¦Â-Lactam antibiotics are the mainstay for the treatment of bacterial infections. However, elevated resistance to these antibiotics mediated by metallo-¦Â-lactamases (MBLs) has become a global concern. New Delhi metallo-¦Â-lactamase-1 (NDM-1), a newly added member of the MBL family that can hydrolyze almost all ¦Â-lactam antibiotics, has rapidly spread all over the world and poses serious clinical threats. Broad-spectrum and mechanism-based inhibitors against all MBLs are highly desired, but the differential mechanisms of MBLs toward different antibiotics pose a great challenge. To facilitate the design of mechanism-based inhibitors, we investigated the active-site conformational changes of NDM-1 through the determination of a series of 15 high-resolution crystal structures in native form and in complex with products and by using biochemical and biophysical studies, site-directed mutagenesis, and molecular dynamics computation. The structural studies reveal the consistency of the active-site conformations in NDM-1/product complexes and the fluctuation in native NDM-1 structures. The enzymatic measurements indicate a correlation between enzymatic activity and the active-site fluctuation, with more fluctuation favoring higher activity. This correlation is further validated by structural and enzymatic studies of the Q123G mutant. Our combinational studies suggest that active-site conformational fluctuation promotes the enzymatic activity of NDM-1, which may guide further mechanism studies and inhibitor design.
Organizational Affiliation:
Department of Biology, Guangdong Provincial Key Laboratory of Cell Microenvironment and Disease Research, Southern University of Science and Technology, Shenzhen, China zhanghm@sustc.edu.cn qhao@hku.hk.