Antibacterial Spectrum of a Tetrazole-Based Reversible Inhibitor of Serine beta-Lactamases.
Pemberton, O.A., Zhang, X., Nichols, D.A., DeFrees, K., Jaishankar, P., Bonnet, R., Adams, J., Shaw, L.N., Renslo, A.R., Chen, Y.(2018) Antimicrob Agents Chemother 62
- PubMed: 29844038
- DOI: https://doi.org/10.1128/AAC.02563-17
- Primary Citation of Related Structures:
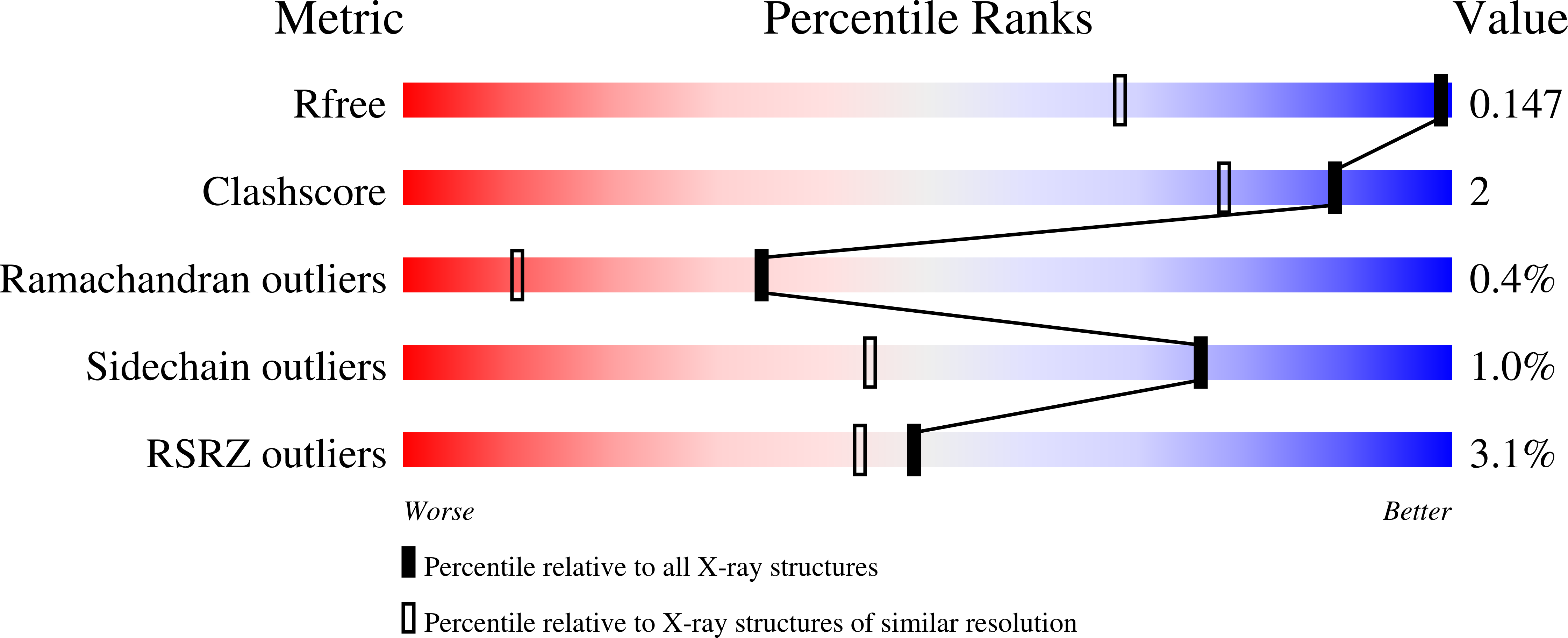
6BT6, 6BU3 - PubMed Abstract:
CTX-M is the most prevalent family of extended-spectrum ¦Â-lactamases. We recently developed a tetrazole-derived noncovalent inhibitor of CTX-M-9. Here, we present the biochemical and microbiological activity of this inhibitor across a representative panel of serine ¦Â-lactamases and Gram-negative bacteria. The compound displayed significant activity against all major subgroups of CTX-M, including CTX-M-15, while it exhibited some low-level inhibition of other serine ¦Â-lactamases. Complex crystal structures with the CTX-M-14 S237A mutant and CTX-M-27 illustrate the binding contribution of specific active-site residues on the ¦Â3 strand. In vitro pharmacokinetic studies revealed drug-like properties and positive prospects for further optimization. These studies suggest that tetrazole-based compounds can provide novel chemotypes for future serine ¦Â-lactamase inhibitor discovery.
Organizational Affiliation:
Department of Molecular Medicine, University of South Florida, Tampa, Florida, USA.