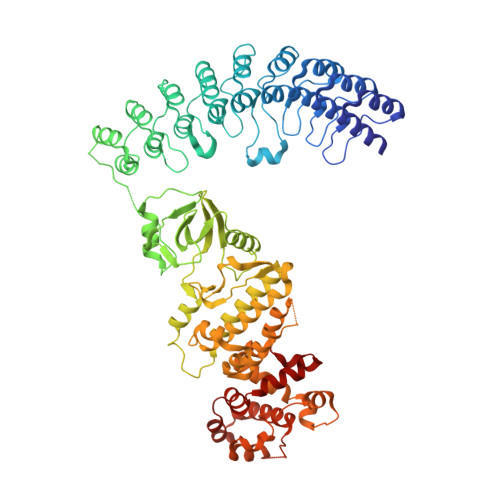
Sunitinib inhibits RNase L by destabilizing its active dimer conformation.
Tang, J., Wang, Y., Zhou, H., Ye, Y., Talukdar, M., Fu, Z., Liu, Z., Li, J., Neculai, D., Gao, J., Huang, H.(2020) Biochem J 477: 3387-3399
- PubMed: 32830849
- DOI: https://doi.org/10.1042/BCJ20200260
- Primary Citation of Related Structures:
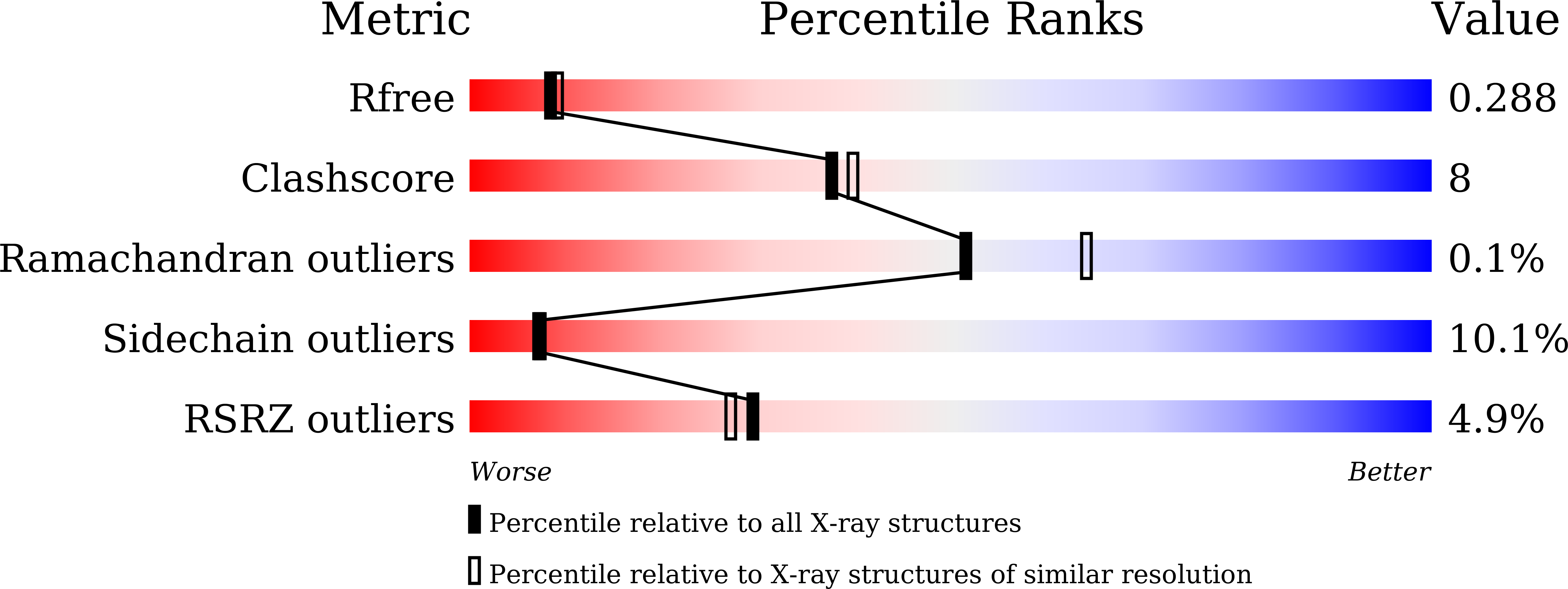
6M11, 6M12, 6M13 - PubMed Abstract:
The pseudokinase (PK) RNase L is a functional ribonuclease and plays important roles in human innate immunity. The ribonuclease activity of RNase L can be regulated by the kinase inhibitor sunitinib. The combined use of oncolytic virus and sunitinib has been shown to exert synergistic effects in anticancer therapy. In this study, we aimed to uncover the mechanism of action through which sunitinib inhibits RNase L. We solved the crystal structures of RNase L in complex with sunitinib and its analogs toceranib and SU11652. Our results showed that sunitinib bound to the ATP-binding pocket of RNase L. Unexpectedly, the ¦ÁA helix linking the ankyrin repeat-domain and the PK domain affected the binding mode of sunitinib and resulted in an unusual flipped orientation relative to other structures in PDB. Molecular dynamics simulations and dynamic light scattering results support that the binding of sunitinib in the PK domain destabilized the dimer conformation of RNase L and allosterically inhibited its ribonuclease activity. Our study suggested that dimer destabilization could be an effective strategy for the discovery of RNase L inhibitors and that targeting the ATP-binding pocket in the PK domain of RNase L was an efficient approach for modulating its ribonuclease activity.
Organizational Affiliation:
State Key Laboratory of Chemical Oncogenomics, School of Chemical Biology and Biotechnology, Peking University Shenzhen Graduate School, Shenzhen, China.