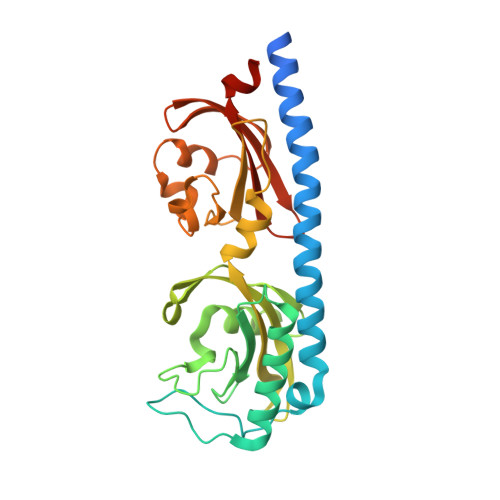
Structure of a double CACHE chemoreceptor ligand-binding domain from Pseudomonas syringae provides insights into the basis of proline recognition.
Ehrhardt, M.K.G., Gerth, M.L., Johnston, J.M.(2021) Biochem Biophys Res Commun 549: 194-199
- PubMed: 33721671
- DOI: https://doi.org/10.1016/j.bbrc.2021.02.090
- Primary Citation of Related Structures:
6MNI - PubMed Abstract:
Chemotaxis is the process of sensing chemical gradients and navigating towards favourable conditions. Bacterial chemotaxis is mediated by arrays of trans-membrane chemoreceptor proteins. The most common class of chemoreceptors have periplasmic ligand-binding domains (LBDs) that detect extracellular chemical signs and transduce these signals to the downstream chemotaxis machinery. The repertoire of chemoreceptor proteins in a bacterium determines the range of environmental signals to which it can respond. Pseudomonas syringae pv. actinidiae (Psa) is a plant pathogen which causes bacterial canker of kiwifruit (Actinidia sp.). Compared to many other bacteria, Psa has a large number of chemoreceptors encoded in its genome (43) and most of these remain uncharacterized. A previous study identified PscC as a potential chemoreceptor for l-proline and other amino acid ligands. Here, we have characterized the interaction of PscC-LBD with l-proline using a combination of isothermal titration calorimetry (ITC) and X-ray crystallography. ITC confirmed direct binding of l-proline to PscC-LBD with K D value of 5.0?¦̀M. We determined the structure of PscC-LBD in complex with l-proline. Our structural analysis showed that PscC-LBD adopts similar double-CACHE fold to several other amino acid chemoreceptors. A comparison of the PscC-LDB to other dCACHE structures highlights residues in the binding cavity which contribute to its ligand specificity.
Organizational Affiliation:
Department of Biochemistry, University of Otago, Dunedin, New Zealand.