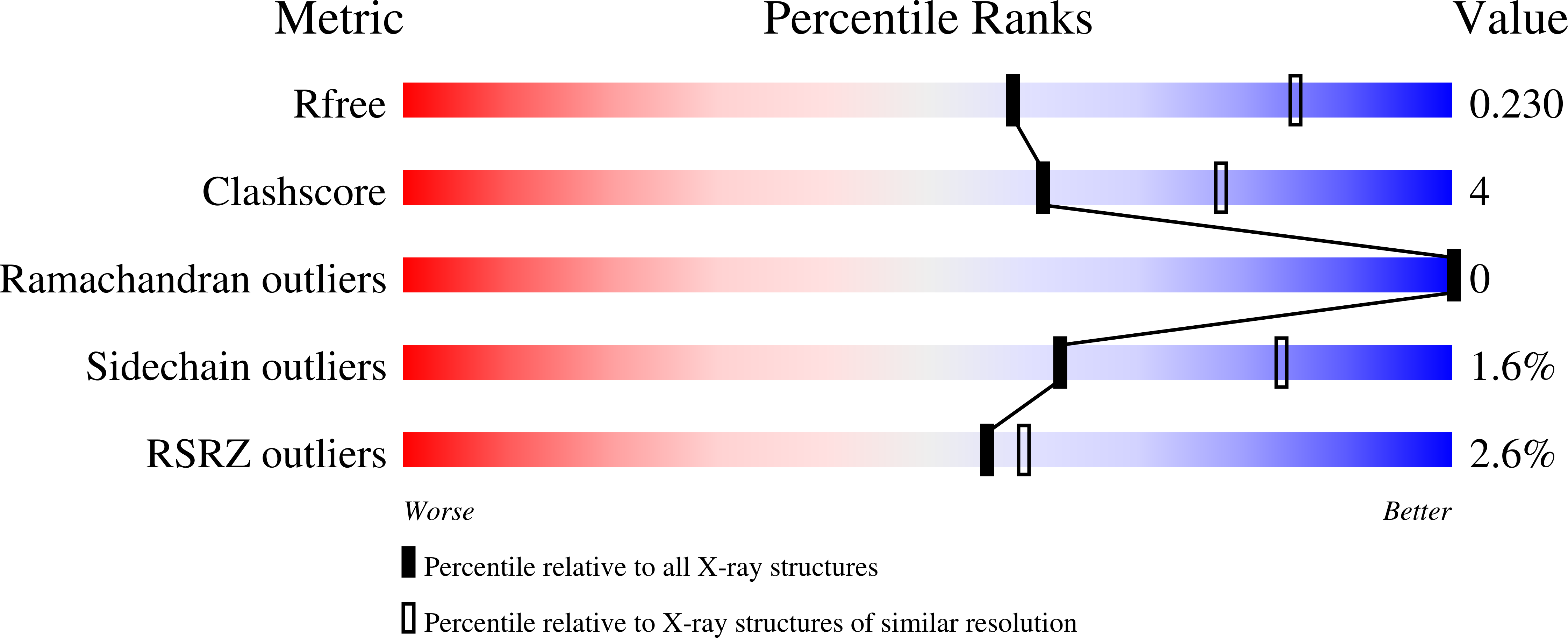
S-3-Carboxypropyl-l-cysteine specifically inhibits cystathionine gamma-lyase-dependent hydrogen sulfide synthesis.
Yadav, P.K., Vitvitsky, V., Kim, H., White, A., Cho, U.S., Banerjee, R.(2019) J Biological Chem 294: 11011-11022
- PubMed: 31160338
- DOI: https://doi.org/10.1074/jbc.RA119.009047
- Primary Citation of Related Structures:
6NBA - PubMed Abstract:
Hydrogen sulfide (H 2 S) is a gaseous signaling molecule, which modulates a wide range of mammalian physiological processes. Cystathionine ¦Ã-lyase (CSE) catalyzes H 2 S synthesis and is a potential target for modulating H 2 S levels under pathophysiological conditions. CSE is inhibited by propargylglycine (PPG), a widely used mechanism-based inhibitor. In this study, we report that inhibition of H 2 S synthesis from cysteine, but not the canonical cystathionine cleavage reaction catalyzed by CSE in vitro , is sensitive to preincubation of the enzyme with PPG. In contrast, the efficacy of S -3-carboxpropyl-l-cysteine (CPC) a new inhibitor described herein, was not dependent on the order of substrate/inhibitor addition. We observed that CPC inhibited the ¦Ã-elimination reaction of cystathionine and H 2 S synthesis from cysteine by human CSE with K i values of 50 ¡À 3 and 180 ¡À 15 ¦Ìm, respectively. We noted that CPC spared the other enzymes involved either directly (cystathionine ¦Â-synthase and mercaptopyruvate sulfurtransferase) or indirectly (cysteine aminotransferase) in H 2 S biogenesis. CPC also targeted CSE in cultured cells, inhibiting transsulfuration flux by 80-90%, as monitored by the transfer of radiolabel from [ 35 S]methionine to GSH. The 2.5 ? resolution crystal structure of human CSE in complex with the CPC-derived aminoacrylate intermediate provided a structural framework for the molecular basis of its inhibitory effect. In summary, our study reveals a previously unknown confounding effect of PPG, widely used to inhibit CSE-dependent H 2 S synthesis, and reports on an alternative inhibitor, CPC, which could be used as a scaffold to develop more potent H 2 S biogenesis inhibitors.
Organizational Affiliation:
Department of Biological Chemistry, University of Michigan Medical School, Ann Arbor, Michigan 48109 and.