Methionine Adenosyltransferase Engineering to Enable Bioorthogonal Platforms for AdoMet-Utilizing Enzymes.
Huber, T.D., Clinger, J.A., Liu, Y., Xu, W., Miller, M.D., Phillips Jr., G.N., Thorson, J.S.(2020) ACS Chem Biol 15: 695-705
- PubMed: 32091873
- DOI: https://doi.org/10.1021/acschembio.9b00943
- Primary Citation of Related Structures:
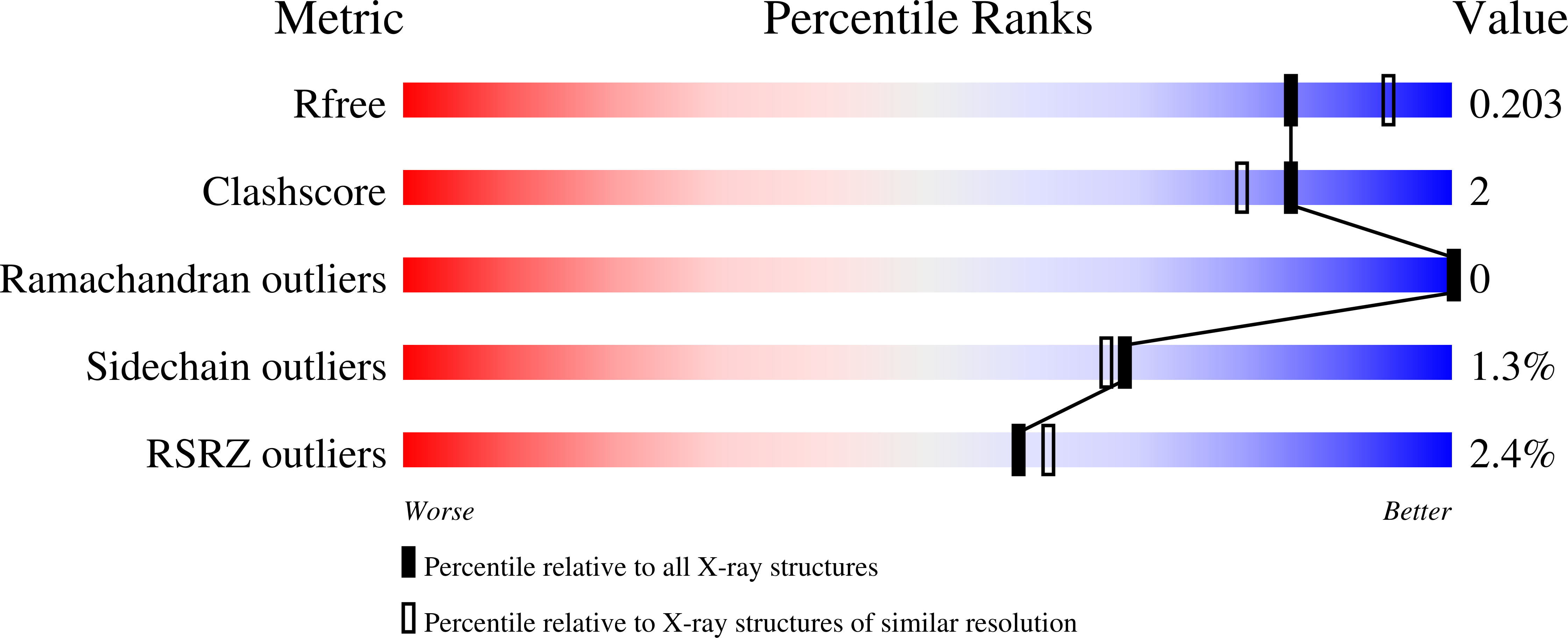
6P9V - PubMed Abstract:
The structural conservation among methyltransferases (MTs) and MT functional redundancy is a major challenge to the cellular study of individual MTs. As a first step toward the development of an alternative biorthogonal platform for MTs and other AdoMet-utilizing enzymes, we describe the evaluation of 38 human methionine adenosyltransferase II-¦Á (hMAT2A) mutants in combination with 14 non-native methionine analogues to identify suitable bioorthogonal mutant/analogue pairings. Enabled by the development and implementation of a hMAT2A high-throughput (HT) assay, this study revealed hMAT2A K289L to afford a 160-fold inversion of the hMAT2A selectivity index for a non-native methionine analogue over the native substrate l-Met. Structure elucidation of K289L revealed the mutant to be folded normally with minor observed repacking within the modified substrate pocket. This study highlights the first example of exchanging l-Met terminal carboxylate/amine recognition elements within the hMAT2A active-site to enable non-native bioorthgonal substrate utilization. Additionally, several hMAT2A mutants and l-Met substrate analogues produced AdoMet analogue products with increased stability. As many AdoMet-producing (e.g., hMAT2A) and AdoMet-utlizing (e.g., MTs) enzymes adopt similar active-site strategies for substrate recognition, the proof of concept first generation hMAT2A engineering highlighted herein is expected to translate to a range of AdoMet-utilizing target enzymes.
Organizational Affiliation:
Department of Pharmaceutical Sciences, College of Pharmacy, University of Kentucky, 789 South Limestone Street, Lexington, Kentucky 40536-0596, United States.