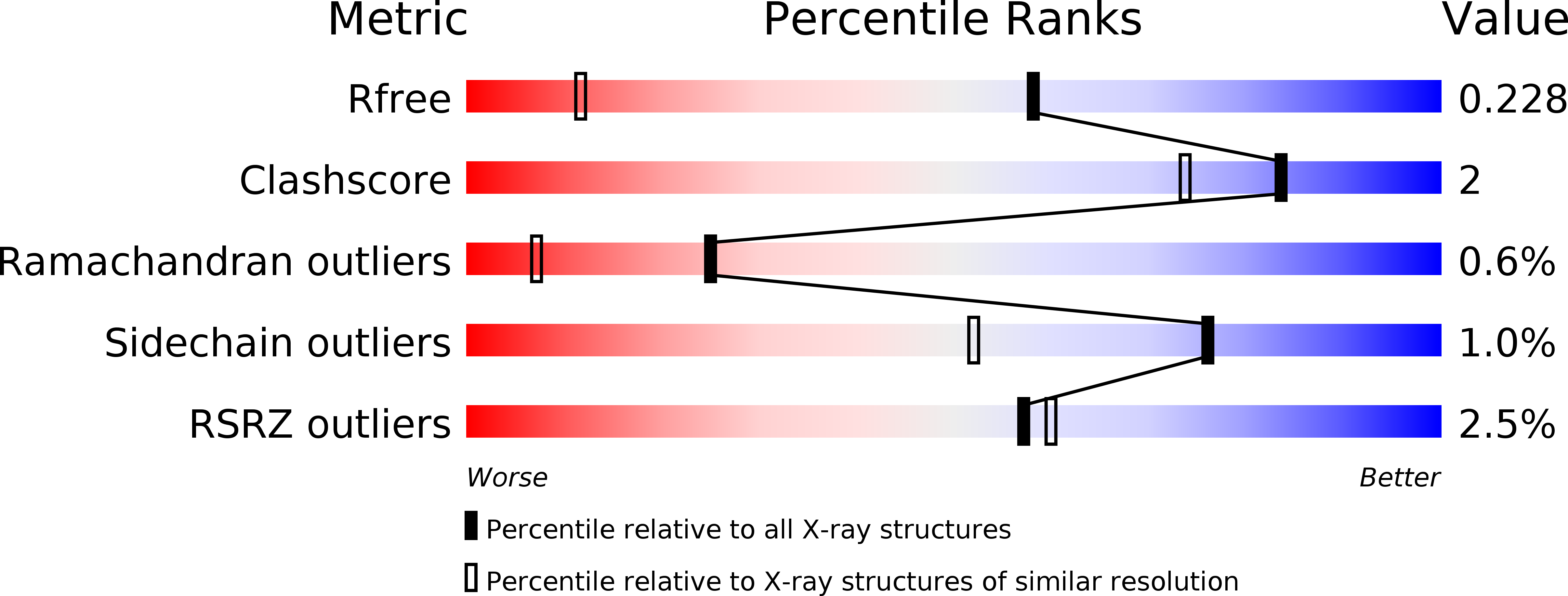
Discovery of 2-phenoxyacetamides as inhibitors of the Wnt-depalmitoleating enzyme NOTUM from an X-ray fragment screen.
Atkinson, B.N., Steadman, D., Zhao, Y., Sipthorp, J., Vecchia, L., Ruza, R.R., Jeganathan, F., Lines, G., Frew, S., Monaghan, A., Kj?r, S., Bictash, M., Jones, E.Y., Fish, P.V.(2019) Medchemcomm 10: 1361-1369
- PubMed: 31534655
- DOI: https://doi.org/10.1039/c9md00096h
- Primary Citation of Related Structures:
6R8P, 6R8Q, 6R8R - PubMed Abstract:
NOTUM is a carboxylesterase that has been shown to act by mediating the O -depalmitoleoylation of Wnt proteins resulting in suppression of Wnt signaling. Here, we describe the development of NOTUM inhibitors that restore Wnt signaling for use in in vitro disease models where NOTUM over activity is an underlying cause. A crystallographic fragment screen with NOTUM identified 2-phenoxyacetamide 3 as binding in the palmitoleate pocket with modest inhibition activity (IC 50 33 ¦̀M). Optimization of hit 3 by SAR studies guided by SBDD identified indazole 38 (IC 50 0.032 ¦̀M) and isoquinoline 45 (IC 50 0.085 ¦̀M) as potent inhibitors of NOTUM. The binding of 45 to NOTUM was rationalized through an X-ray co-crystal structure determination which showed a flipped binding orientation compared to 3 . However, it was not possible to combine NOTUM inhibition activity with metabolic stability as the majority of the compounds tested were rapidly metabolized in an NADPH-independent manner.
Organizational Affiliation:
Alzheimer's Research UK UCL Drug Discovery Institute , University College London , Cruciform Building, Gower Street , London , WC1E 6BT , UK . Email: p.fish@ucl.ac.uk ; Tel: +44 (0)20 7679 6971.