Structural and in Vivo Characterization of Tubastatin A, a Widely Used Histone Deacetylase 6 Inhibitor.
Shen, S., Svoboda, M., Zhang, G., Cavasin, M.A., Motlova, L., McKinsey, T.A., Eubanks, J.H., Barinka, C., Kozikowski, A.P.(2020) ACS Med Chem Lett 11: 706-712
- PubMed: 32435374
- DOI: https://doi.org/10.1021/acsmedchemlett.9b00560
- Primary Citation of Related Structures:
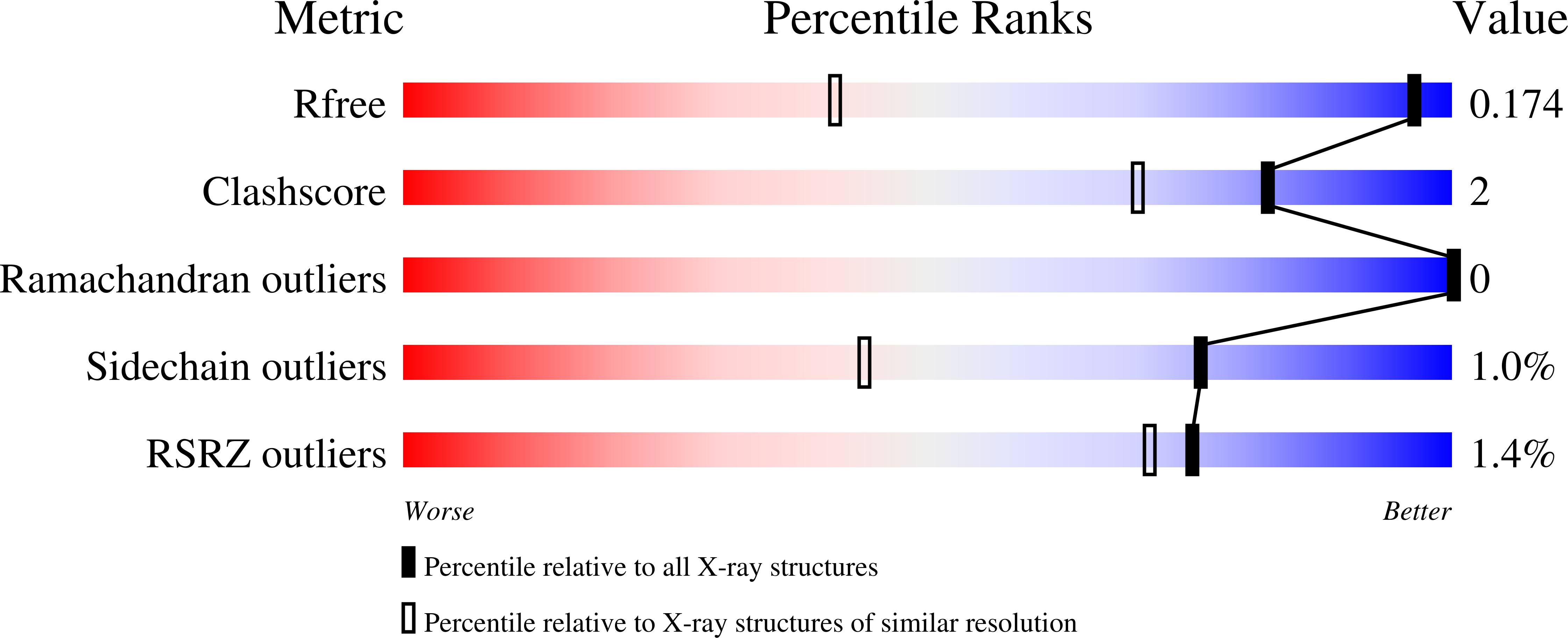
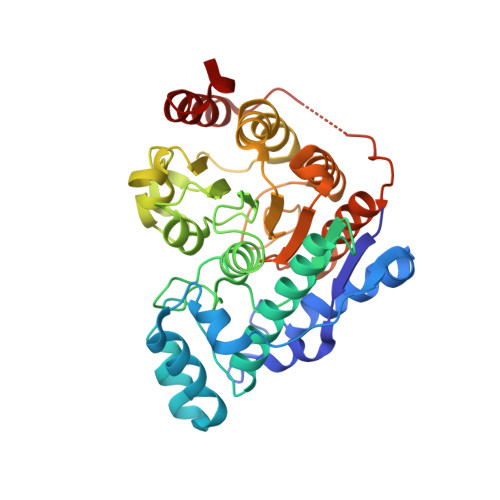
6THV - PubMed Abstract:
Tubastatin A, a tetrahydro-¦Ă-carboline-capped selective HDAC6 inhibitor (HDAC6i), was rationally designed 10 years ago, and has become the best investigated HDAC6i to date. It shows efficacy in various neurological disease animal models, as HDAC6 plays a crucial regulatory role in axonal transport deficits, protein aggregation, as well as oxidative stress. In this work, we provide new insights into this HDAC6i by investigating the molecular basis of its interactions with HDAC6 through X-ray crystallography, determining its functional capability to elevate the levels of acetylated ¦Á-tubulin in vitro and in vivo, correlating PK/PD profiles to determine effective doses in plasma and brain, and finally assessing its therapeutic potential toward psychiatric diseases through use of the SmartCube screening platform.
Organizational Affiliation:
Department of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, University of Illinois at Chicago, Chicago, Illinois 60612, United States.