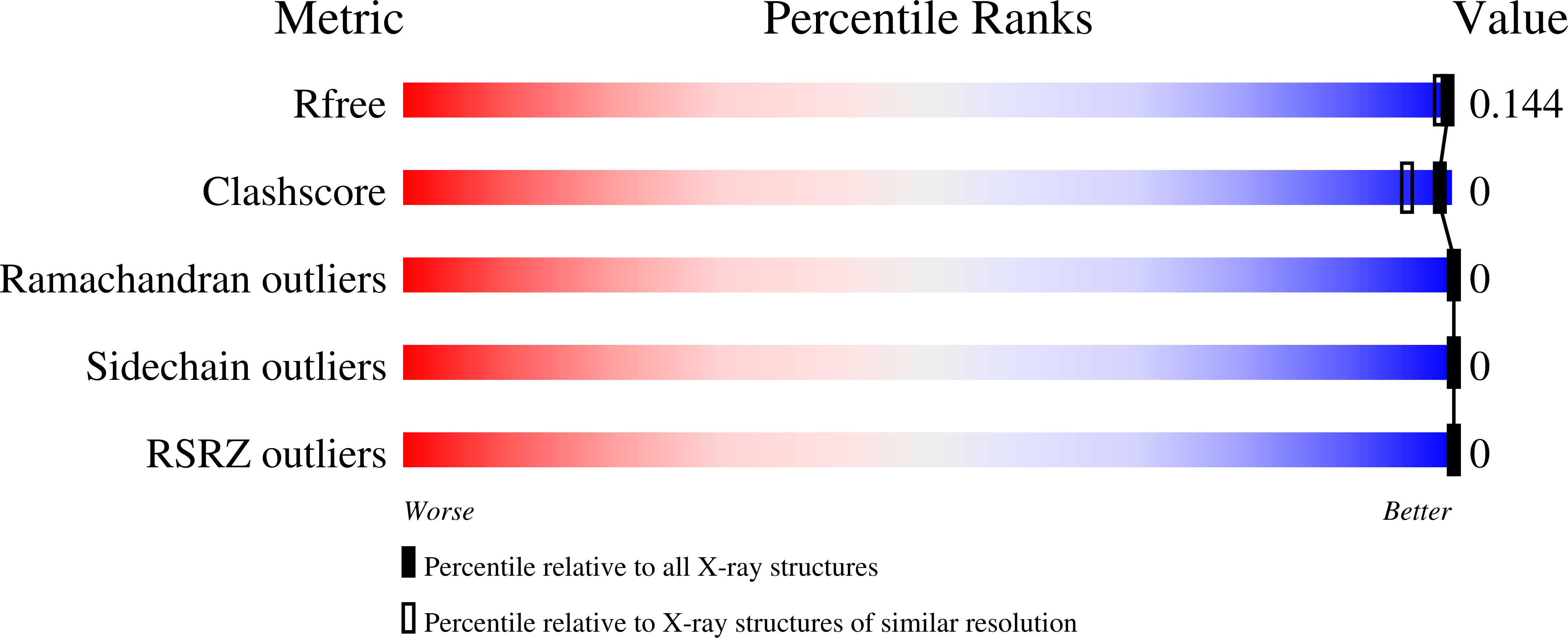
Crystal structures of SARS-CoV-2 ADP-ribose phosphatase: from the apo form to ligand complexes.
Michalska, K., Kim, Y., Jedrzejczak, R., Maltseva, N.I., Stols, L., Endres, M., Joachimiak, A.(2020) IUCrJ 7: 814-824
- PubMed: 32939273
- DOI: https://doi.org/10.1107/S2052252520009653
- Primary Citation of Related Structures:
6VXS, 6W02, 6W6Y, 6WCF, 6WEN - PubMed Abstract:
Among 15 nonstructural proteins (Nsps), the newly emerging Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2) encodes a large, multidomain Nsp3. One of its units is the ADP-ribose phosphatase domain (ADRP; also known as the macrodomain, MacroD), which is believed to interfere with the host immune response. Such a function appears to be linked to the ability of the protein to remove ADP-ribose from ADP-ribosylated proteins and RNA, yet the precise role and molecular targets of the enzyme remain unknown. Here, five high-resolution (1.07-2.01??) crystal structures corresponding to the apo form of the protein and its complexes with 2-( N -morpholino)ethanesulfonic acid (MES), AMP and ADP-ribose have been determined. The protein is shown to undergo conformational changes to adapt to the ligand in the manner previously observed in close homologues from other viruses. A conserved water molecule is also identified that may participate in hydrolysis. This work builds foundations for future structure-based research on ADRP, including the search for potential antiviral therapeutics.
Organizational Affiliation:
Center for Structural Genomics of Infectious Diseases, Consortium for Advanced Science and Engineering, University of Chicago, Chicago, IL 60667, USA.