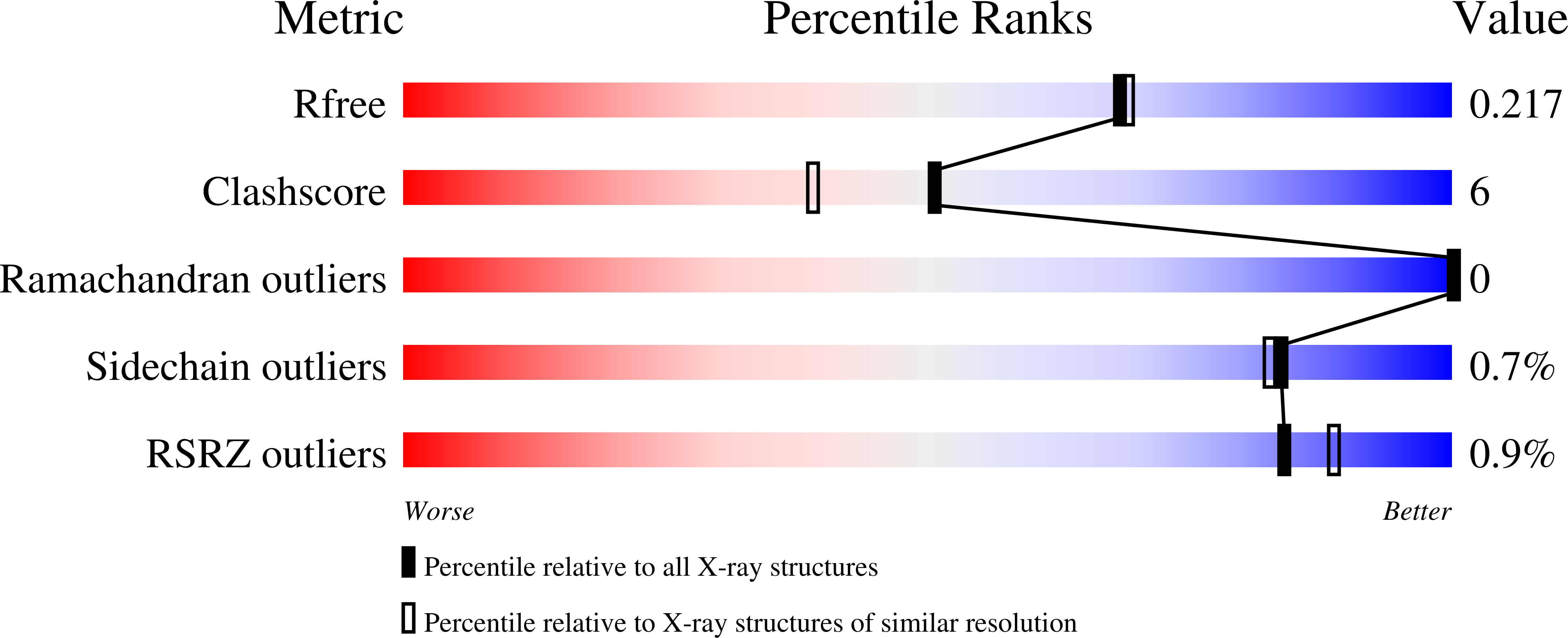
A family of radical halogenases for the engineering of amino-acid-based products.
Neugebauer, M.E., Sumida, K.H., Pelton, J.G., McMurry, J.L., Marchand, J.A., Chang, M.C.Y.(2019) Nat Chem Biol 15: 1009-1016
- PubMed: 31548692
- DOI: https://doi.org/10.1038/s41589-019-0355-x
- Primary Citation of Related Structures:
6NIE - PubMed Abstract:
The integration of synthetic and biological catalysis enables new approaches to the synthesis of small molecules by combining the high selectivity of enzymes with the reaction diversity offered by synthetic chemistry. While organohalogens are valued for their bioactivity and utility as synthetic building blocks, only a handful of enzymes that carry out the regioselective halogenation of unactivated [Formula: see text] bonds have previously been identified. In this context, we report the structural characterization of BesD, a recently discovered radical halogenase from the Fe II /¦Á-ketogluturate-dependent family that chlorinates the free amino acid lysine. We also identify and characterize additional halogenases that produce mono- and dichlorinated, as well as brominated and azidated, amino acids. The substrate selectivity of this new family of radical halogenases takes advantage of the central role of amino acids in metabolism and enables engineering of biosynthetic pathways to afford a wide variety of compound classes, including heterocycles, diamines, ¦Á-keto acids and peptides.
Organizational Affiliation:
Department of Chemical and Biomolecular Engineering, University of California, Berkeley, Berkeley, CA, USA.