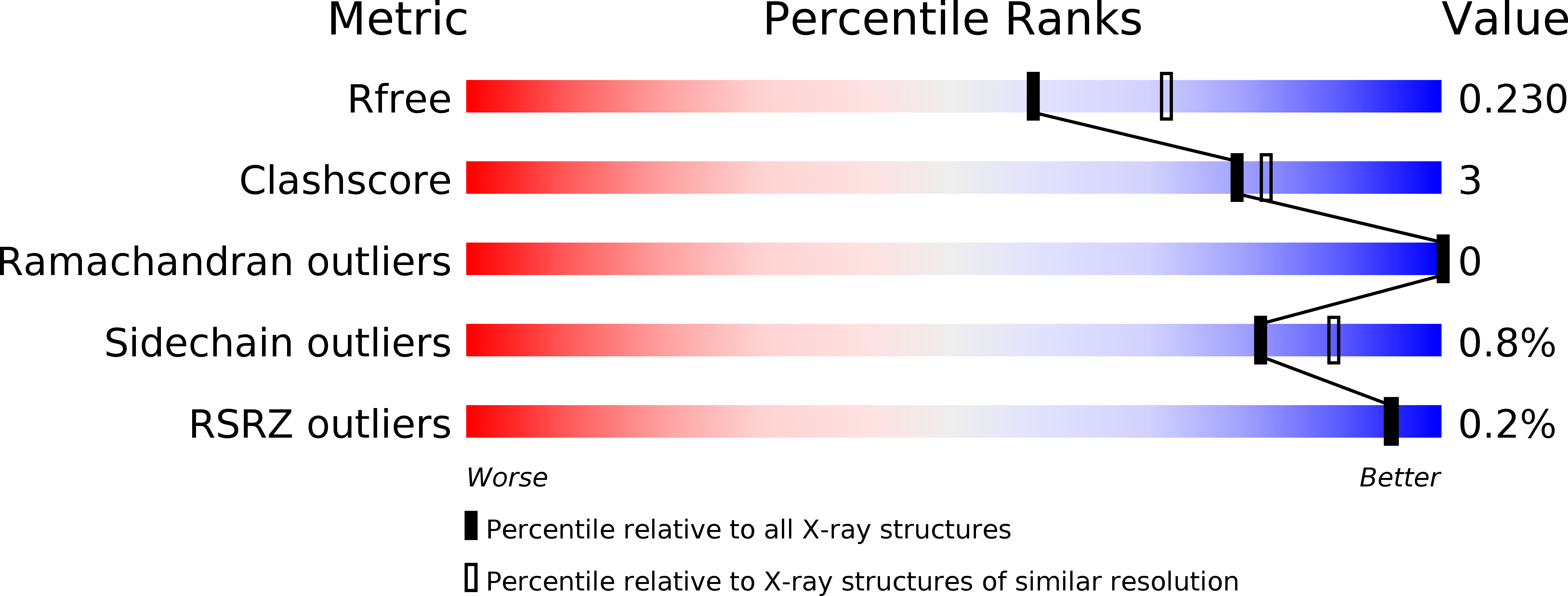
Bicyclic Boronate VNRX-5133 Inhibits Metallo- and Serine-beta-Lactamases.
Krajnc, A., Brem, J., Hinchliffe, P., Calvopina, K., Panduwawala, T.D., Lang, P.A., Kamps, J.J.A.G., Tyrrell, J.M., Widlake, E., Saward, B.G., Walsh, T.R., Spencer, J., Schofield, C.J.(2019) J Med Chem 62: 8544-8556
- PubMed: 31454231
- DOI: https://doi.org/10.1021/acs.jmedchem.9b00911
- Primary Citation of Related Structures:
6RMF, 6RTN - PubMed Abstract:
The bicyclic boronate VNRX-5133 (taniborbactam) is a new type of ¦Â-lactamase inhibitor in clinical development. We report that VNRX-5133 inhibits serine-¦Â-lactamases (SBLs) and some clinically important metallo-¦Â-lactamases (MBLs), including NDM-1 and VIM-1/2. VNRX-5133 activity against IMP-1 and tested B2/B3 MBLs was lower/not observed. Crystallography reveals how VNRX-5133 binds to the class D SBL OXA-10 and MBL NDM-1. The crystallographic results highlight the ability of bicyclic boronates to inhibit SBLs and MBLs via binding of a tetrahedral (sp 3 ) boron species. The structures imply conserved binding of the bicyclic core with SBLs/MBLs. With NDM-1, by crystallography, we observed an unanticipated VNRX-5133 binding mode involving cyclization of its acylamino oxygen onto the boron of the bicyclic core. Different side-chain binding modes for bicyclic boronates for SBLs and MBLs imply scope for side-chain optimization. The results further support the "high-energy-intermediate" analogue approach for broad-spectrum ¦Â-lactamase inhibitor development and highlight the ability of boron inhibitors to interchange between different hybridization states/binding modes.
Organizational Affiliation:
Chemistry Research Laboratory, Department of Chemistry , University of Oxford , 12 Mansfield Road , Oxford OX1 3TA , United Kingdom.