Structural basis for the design of bisubstrate inhibitors of protein kinase CK2 provided by complex structures with the substrate-competitive inhibitor heparin.
Schnitzler, A., Niefind, K.(2021) Eur J Med Chem 214: 113223-113223
- PubMed: 33571828
- DOI: https://doi.org/10.1016/j.ejmech.2021.113223
- Primary Citation of Related Structures:
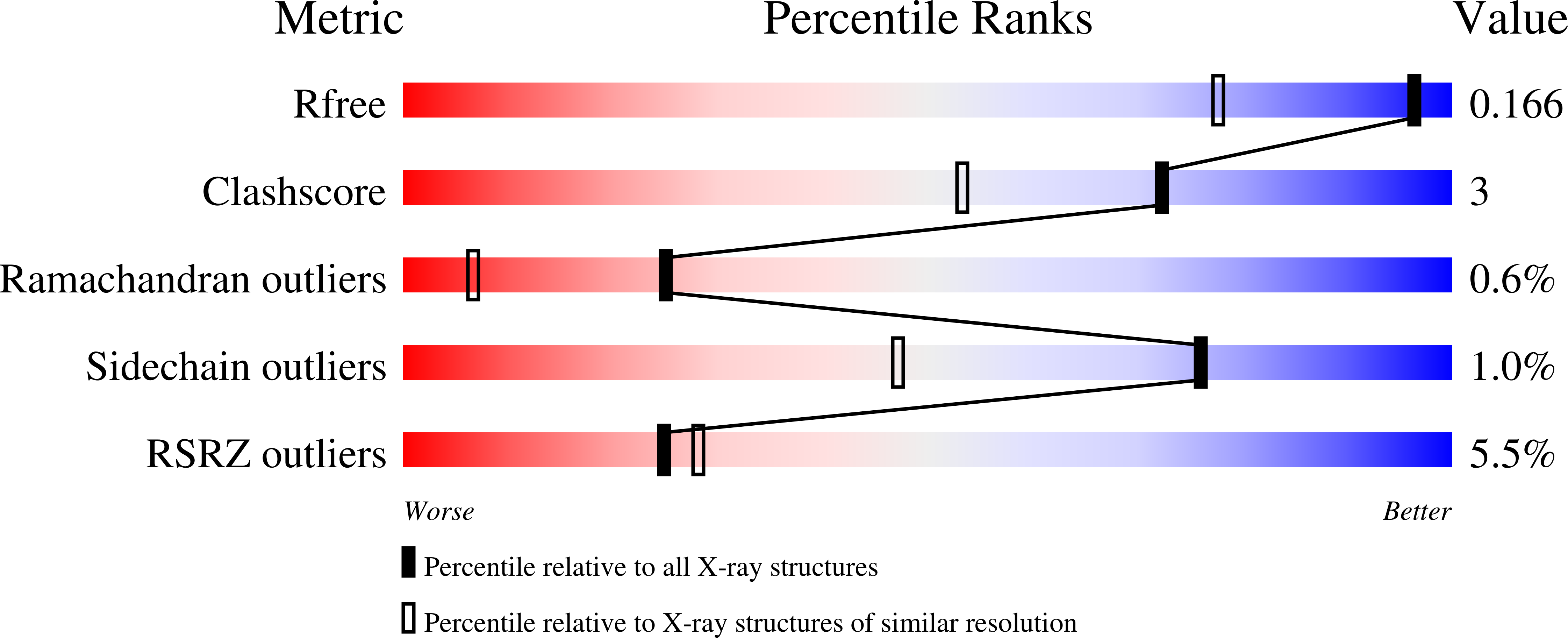
7B8H, 7B8I - PubMed Abstract:
The Ser/Thr kinase CK2, a member of the superfamily of eukaryotic protein kinases, has an acidophilic substrate profile with the substrate recognition sequence S/T-D/E-X-D/E, and it is inhibited by polyanionic substances like heparin. The latter, a highly sulphated glucosamino glycan composed mainly of repeating 2-O-sulpho-¦Á-l-idopyranuronic acid/N,O6-disulpho-¦Á-d-glucosamine disaccharide units, is the longest known substrate-competitive CK2 inhibitor. The structural basis of CK2's preference for anionic substrates and substrate-competitive inhibitors is only vaguely known which limits the value of the substrate-binding region for the structure-based development of CK2 bisubstrate inhibitors. Here, a tetragonal and a monoclinic co-crystal structure of CK2¦Á, the catalytic subunit of CK2, with a decameric heparin fragment are described. In the tetragonal structure, the heparin molecule binds to the polybasic stretch at the beginning of CK2¦Á's helix ¦ÁC, whereas in the monoclinic structure it occupies the central substrate-recognition region around the P+1 loop. Together, the structures rationalize the inhibitory efficacy of heparin fragments as a function of chain length. The monoclinic CK2¦Á/heparin structure, in which the heparin fragment is particularly well defined, is the first CK2 structure with an anionic inhibitor of considerable size at the central part of the substrate-recognition site. The bound heparin fragment is so close to the binding site of ATP-competitive inhibitors that it can guide the design of linkers and pave the way to efficient CK2 bisubstrate inhibitors in the future.
Organizational Affiliation:
Universit?t zu K?ln, Department f¨¹r Chemie, Institut f¨¹r Biochemie, Z¨¹lpicher Stra?e 47, D-50674 K?ln, Germany.