Characterization and structure of glyceraldehyde-3-phosphate dehydrogenase type 1 from Escherichia coli.
Zhang, L., Liu, M.R., Yao, Y.C., Bostrom, I.K., Wang, Y.D., Chen, A.Q., Li, J.X., Gu, S.H., Ji, C.N.(2020) Acta Crystallogr F Struct Biol Commun 76: 406-413
- PubMed: 32880588
- DOI: https://doi.org/10.1107/S2053230X20010067
- Primary Citation of Related Structures:
7C5F - PubMed Abstract:
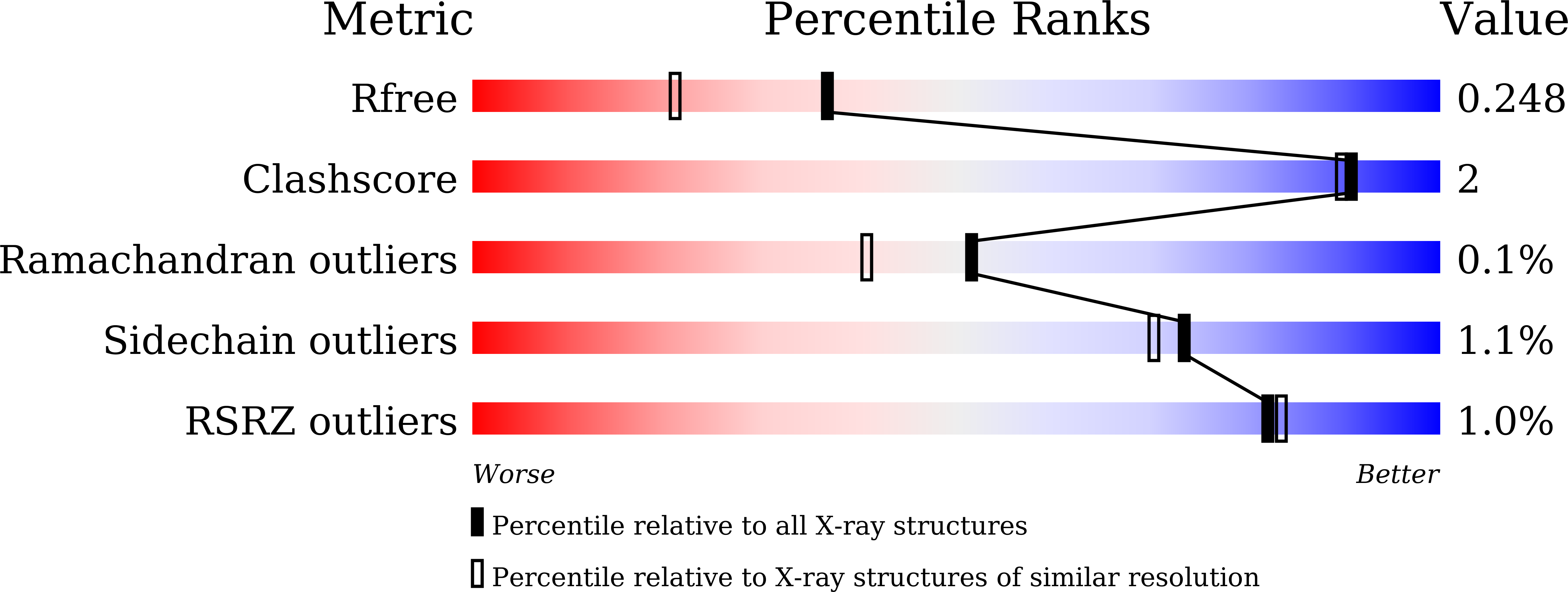
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is a key enzyme in the glycolytic pathway that catalyzes the conversion of D-glyceraldehyde 3-phosphate to 1,3-diphosphoglycerate. Here, the full-length GAPDH type 1 from Escherichia coli (EcGAPDH1) was cloned and overexpressed, and the protein was purified. Biochemical analyses found that the optimum reaction temperature and pH of EcGAPDH1 were 55¡ãC and 10.0, respectively. The protein has a certain amount of thermostability. Crystals of EcGAPDH1 were obtained using the sitting-drop vapor-diffusion technique and X-ray diffraction data were collected to 1.88?? resolution. Characterization of the crystals showed that they belonged to space group P4 1 2 1 2, with unit-cell parameters a = b = 89.651, c?=?341.007??, ¦Á = ¦Â = ¦Ã = 90¡ã. The structure of EcGAPDH1 contains four subunits, each of which includes an N-terminal NAD + -binding domain and a C-terminal catalytic domain. Analysis of the NAD + -bound form showed some differences between the structures of EcGAPDH1 and human GAPDH. As EcGAPDH1 shares 100% identity with GAPDH from Shigella sonnei, its structure may help in finding a drug for the treatment of shigellosis.
Organizational Affiliation:
State Key Laboratory of Genetic Engineering, Institute of Genetics, School of Life Sciences, Fudan University, 2005 Songhu Road, Shanghai 200438, People's Republic of China.