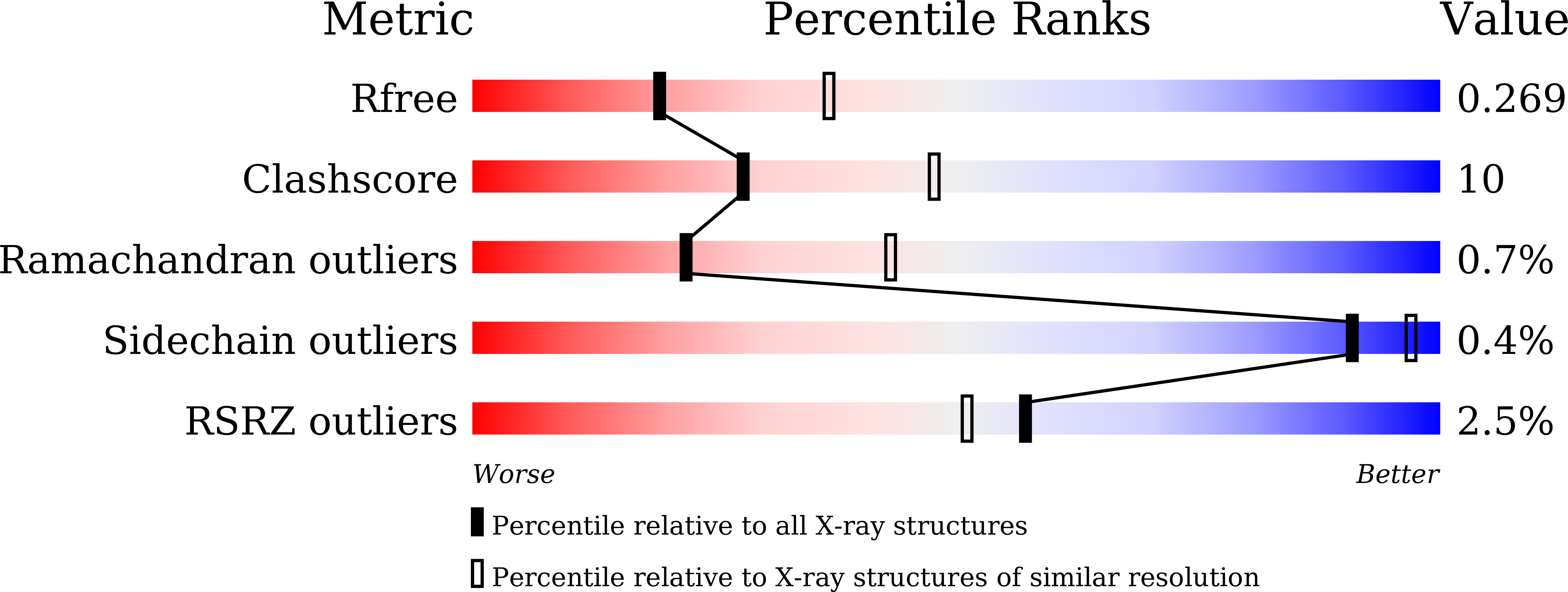
Structure of human ORP3 ORD reveals conservation of a key function and ligand specificity in OSBP-related proteins.
Tong, J., Tan, L., Im, Y.J.(2021) PLoS One 16: e0248781-e0248781
- PubMed: 33857182
- DOI: https://doi.org/10.1371/journal.pone.0248781
- Primary Citation of Related Structures:
7DEI, 7DEJ - PubMed Abstract:
Human ORP3 belongs to the oxysterol-binding protein (OSBP) family of lipid transfer proteins and is involved in lipid trafficking and cell signaling. ORP3 localizes to the ER-PM interfaces and is implicated in lipid transport and focal adhesion dynamics. Here, we report the 2.6-2.7 ? structures of the ORD (OSBP-related domain) of human ORP3 in apo-form and in complex with phosphatidylinositol 4-phosphate. The ORP3 ORD displays a helix grip ¦Â-barrel fold with a deep hydrophobic pocket which is conserved in the OSBP gene family. ORP3 binds PI(4)P by the residues around tunnel entrance and in the hydrophobic pocket, whereas it lacks sterol binding due to the narrow hydrophobic tunnel. The heterologous expression of the ORDs of human ORP3 or OSBP1 rescued the lethality of seven ORP (yeast OSH1-OSH7) knockout in yeast. In contrast, the PI(4)P-binding site mutant of ORP3 did not complement the OSH knockout cells. The N-terminal PH domain and FFAT motif of ORP3 are involved in protein targeting but are not essential in yeast complementation. This observation suggests that the essential function conserved in the ORPs of yeast and human is mediated by PI(4)P-binding of the ORD domain. This study suggests that the non-vesicular PI(4)P transport is a conserved function of all ORPs in eukaryotes.
Organizational Affiliation:
College of Pharmacy, Chonnam National University, Gwangju, Republic of Korea.