Structural basis to repurpose boron-based proteasome inhibitors Bortezomib and Ixazomib as beta-lactamase inhibitors.
Perbandt, M., Werner, N., Prester, A., Rohde, H., Aepfelbacher, M., Hinrichs, W., Betzel, C.(2022) Sci Rep 12: 5510-5510
- PubMed: 35365689
- DOI: https://doi.org/10.1038/s41598-022-09392-6
- Primary Citation of Related Structures:
7Q0Y, 7Q0Z, 7Q11 - PubMed Abstract:
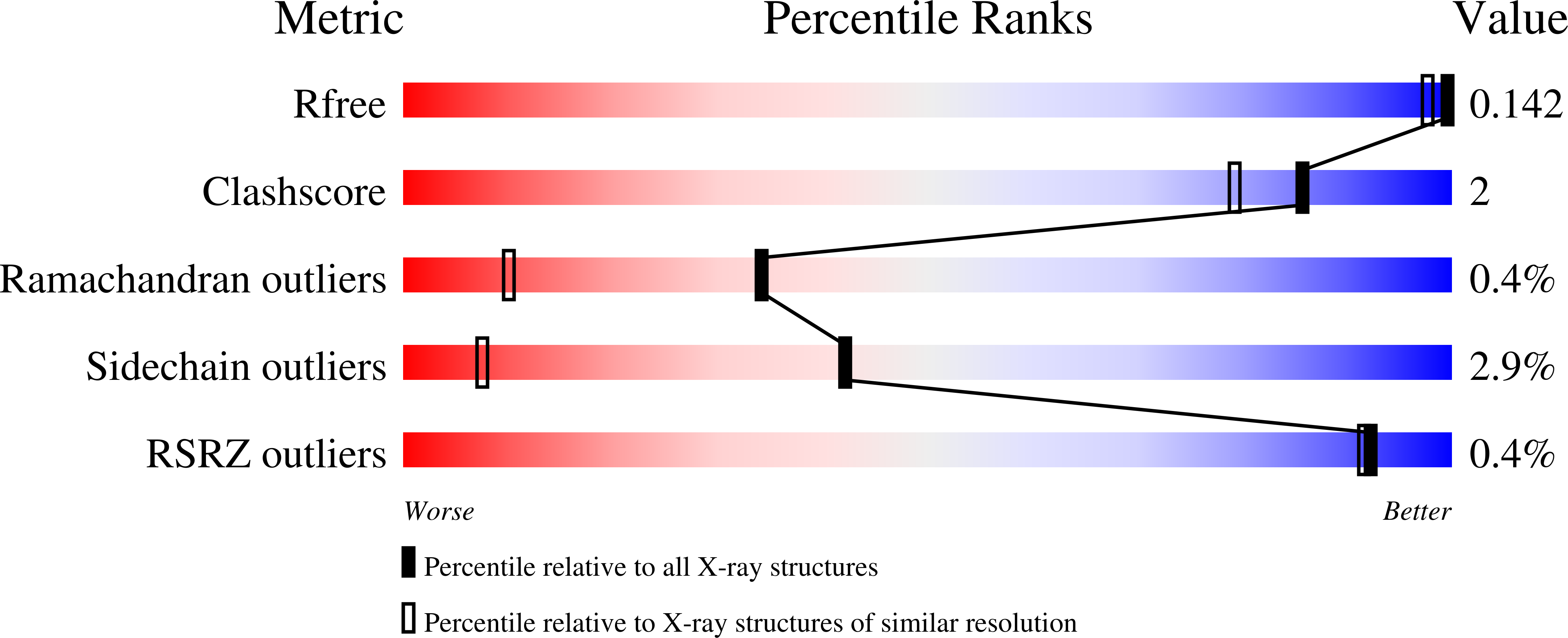
¦Â-lactamases are a major cause of rapidly emerging and spreading antibiotic resistance. Currently ¦Â-lactamase inhibitors (BLIs) in clinical use act only on Ambler Class A, C and some class D lactamases. The urgent need to identify new BLIs recently lead to FDA approval of boron-based compounds BLIs, e.g. Vaborbactam. The boron-based proteasome inhibitors Bortezomib and Ixazomib are used in cancer therapy as multiple myeloma drugs but they also bind to Ser-/Thr- proteases. In this study we show the crystal structures of the ¦Â-lactamase CTX-M-14 with covalently bound Bortezomib and Ixazomib at high resolutions of 1.3 and 1.1??, respectively. Ixazomib is well defined in electron density whereas Bortezomib show some disorder which corresponds to weaker inhibition efficiency observed for Ixazomib. Both inhibitors mimic the deacylation transition state of ¦Â-lactam hydrolysis, because they replace the deacylating water molecule. We further investigate differences in binding of Bortezomib/Ixazomib to CTX-M-14 and its target proteases as well as known ¦Â-lactamase drugs. Our findings can help to use Bortezomib/Ixazomib as lead compounds for development of new BLIs.
Organizational Affiliation:
Institute for Biochemistry and Molecular Biology, University of Hamburg, Hamburg, Germany. markus.perbandt@uni-hamburg.de.