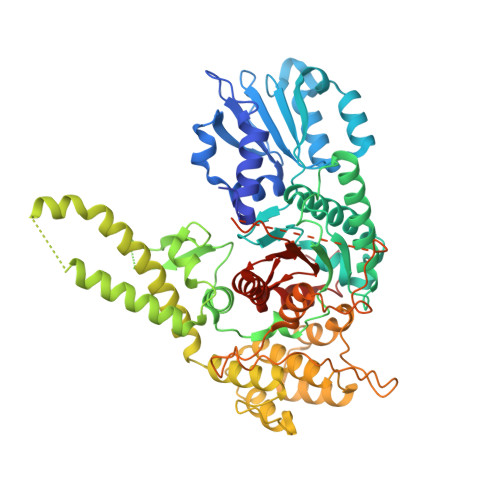
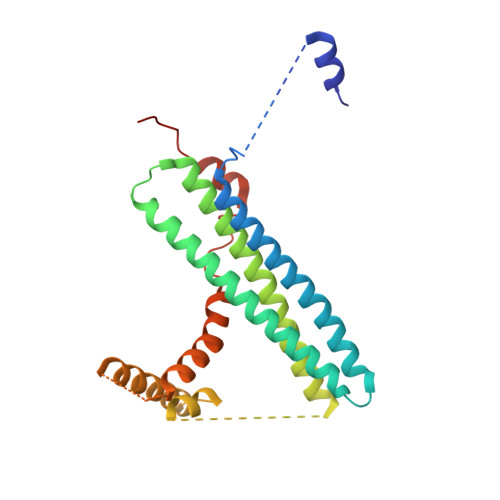
SNARE assembly enlightened by cryo-EM structures of a synaptobrevin-Munc18-1-syntaxin-1 complex.
Stepien, K.P., Xu, J., Zhang, X., Bai, X.C., Rizo, J.(2022) Sci Adv 8: eabo5272-eabo5272
- PubMed: 35731863
- DOI: https://doi.org/10.1126/sciadv.abo5272
- Primary Citation of Related Structures:
7UDB, 7UDC - PubMed Abstract:
Munc18-1 forms a template to organize assembly of the neuronal SNARE complex that triggers neurotransmitter release, binding first to a closed conformation of syntaxin-1 where its amino-terminal region interacts with the SNARE motif, and later binding to synaptobrevin. However, the mechanism of SNARE complex assembly remains unclear. Here, we report two cryo-EM structures of Munc18-1 bound to cross-linked syntaxin-1 and synaptobrevin. The structures allow visualization of how syntaxin-1 opens and reveal how part of the syntaxin-1 amino-terminal region can help nucleate interactions between the amino termini of the syntaxin-1 and synaptobrevin SNARE motifs, while their carboxyl termini bind to distal sites of Munc18-1. These observations, together with mutagenesis, SNARE complex assembly experiments, and fusion assays with reconstituted proteoliposomes, support a model whereby these interactions are critical to initiate SNARE complex assembly and multiple energy barriers enable diverse mechanisms for exquisite regulation of neurotransmitter release.
Organizational Affiliation:
Department of Biophysics, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA.