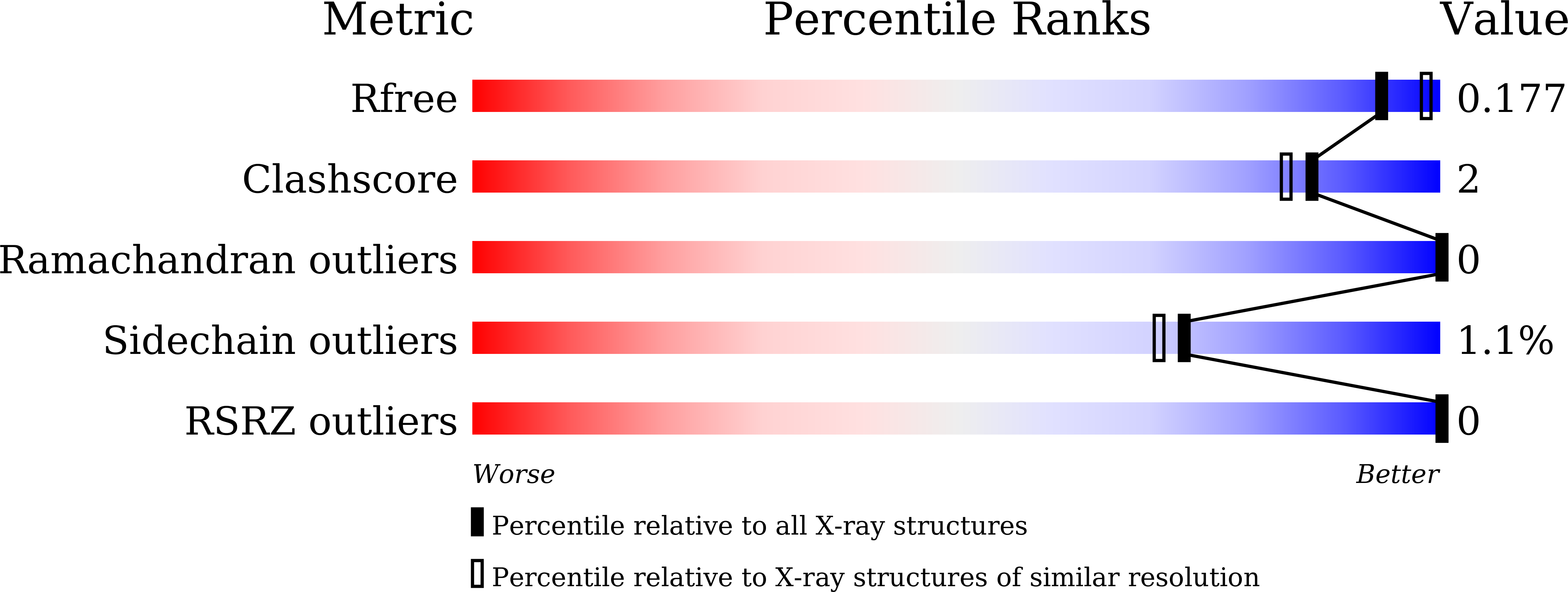
Phenylalanine stacking enhances the red fluorescence of biliverdin IX alpha on UV excitation in sandercyanin fluorescent protein.
Yadav, K., Ghosh, S., Barak, A., Schaefer, W., Subramanian, R.(2022) FEBS Lett 596: 796-805
- PubMed: 35020202
- DOI: https://doi.org/10.1002/1873-3468.14281
- Primary Citation of Related Structures:
7VNL, 7VNS - PubMed Abstract:
Biliverdin IX¦Á (BV) binds to several prokaryotic and eukaryotic proteins. How nature exploits the versatility of BV's properties is not fully understood. Unlike free BV, the Sandercyanin fluorescent protein bound to BV (SFP-BV) shows enhanced red fluorescence (675?nm) on excitation in the UV region (380?nm). Site-directed mutagenesis showed that the BV complex of two SFP variants, F55A and E79A, resulted in the loss of red fluorescence. Crystal structures of the complexes of these proteins with BV show the absence of stacking interactions of the F55 phenyl ring with BV. BV changes from ZZZssa conformation in the wild-type to ZZZsss conformation in the variants. In the nonfluorescent mutants, the lowest excited state is destabilized, resulting in nonradiative decay.
Organizational Affiliation:
Institute for Stem Cell Science and Regenerative Medicine, Bangalore, India.