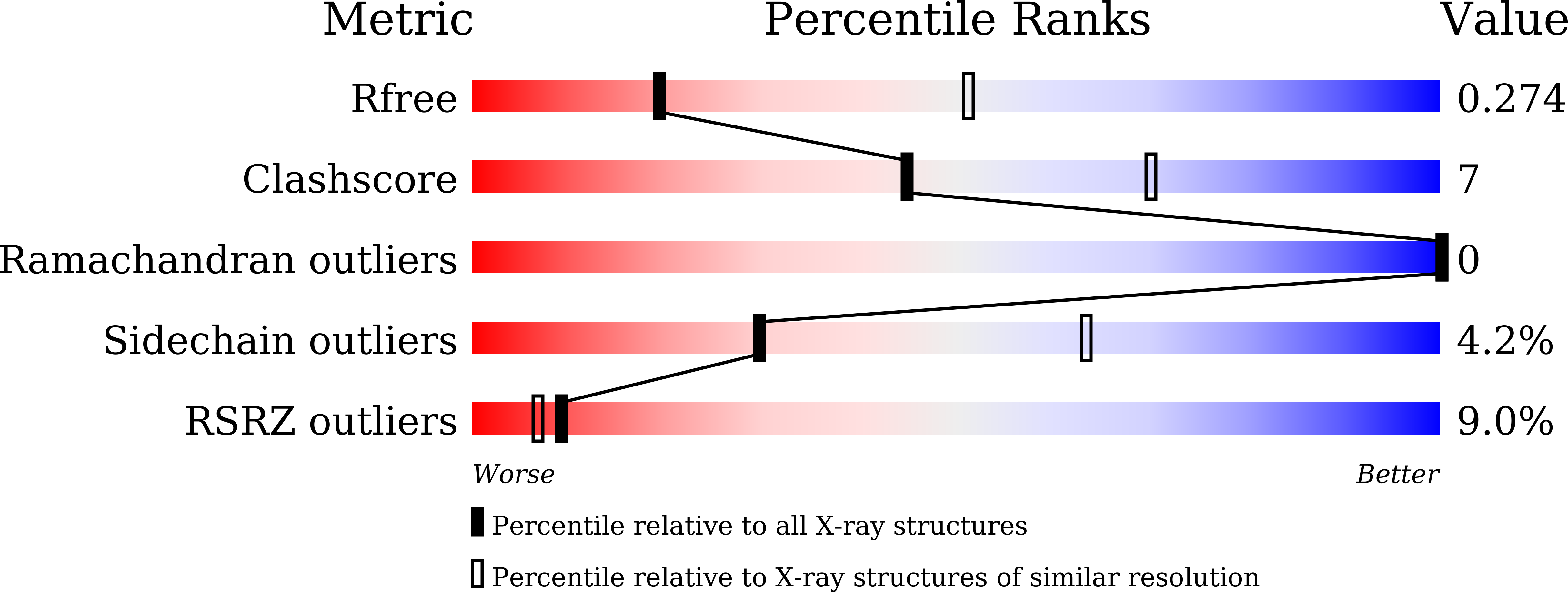
Crystallographic analysis of interaction between cisplatin and human serum albumin: Effect of fatty acid.
Chen, S.L., Yuan, C., Jiang, L.G., Luo, Z.P., Huang, M.D.(2022) Int J Biol Macromol 216: 172-178
- PubMed: 35788007
- DOI: https://doi.org/10.1016/j.ijbiomac.2022.06.181
- Primary Citation of Related Structures:
7WOJ, 7WOK - PubMed Abstract:
Metallodrugs are important for anticancer treatments. They bind mainly to human serum albumin (HSA) in blood circulation, greatly modulating their pharmacokinetics and anticancer efficacy. Fatty acid (FA) is one of the most important endogenous ligands of HSA with tight binding to HSA and affecting its conformation. However, the effect of fatty acids on metallodrugs interaction with HSA is unknown. Here we identify the binding sites of a widely used metallodrug, cisplatin, in HSA in the presence or absence of a representative fatty acid, myristate, by X-ray crystallography. Our crystal structures indicate that the sidechain of residue Met548 becomes more exposed to solvent in the presence of fatty acid, and is the main Pt binding site together with Met329 in HSA:Myr:cisplatin ternary structure. An undoubted new Pt binding site is detected at His338 in the presence of fatty acid, and additional two sites are also identified at His146 and His440?+?K436. In addition, we revealed the mechanism of cisplatin-induced HSA aggregation, which is due to the crosslinking between Met298 and His510 of two HSA molecules.
Organizational Affiliation:
College of Chemistry, Fuzhou University, Fuzhou, Fujian 350108, China.