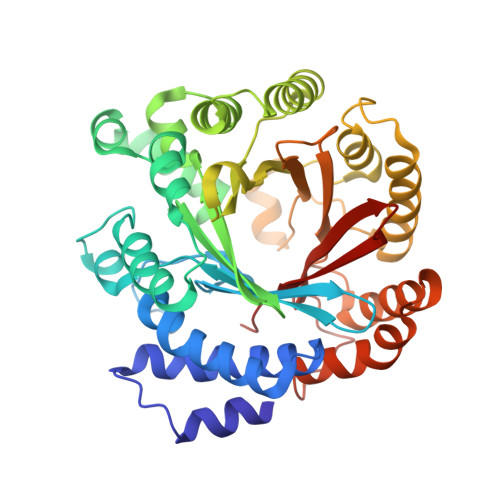
Enzymatic formation of a prenyl beta-carboline by a fungal indole prenyltransferase.
Hamdy, S.A., Kodama, T., Nakashima, Y., Han, X., Matsui, T., Morita, H.(2022) J Nat Med 76: 873-879
- PubMed: 35767141
- DOI: https://doi.org/10.1007/s11418-022-01635-0
- Primary Citation of Related Structures:
7XVJ - PubMed Abstract:
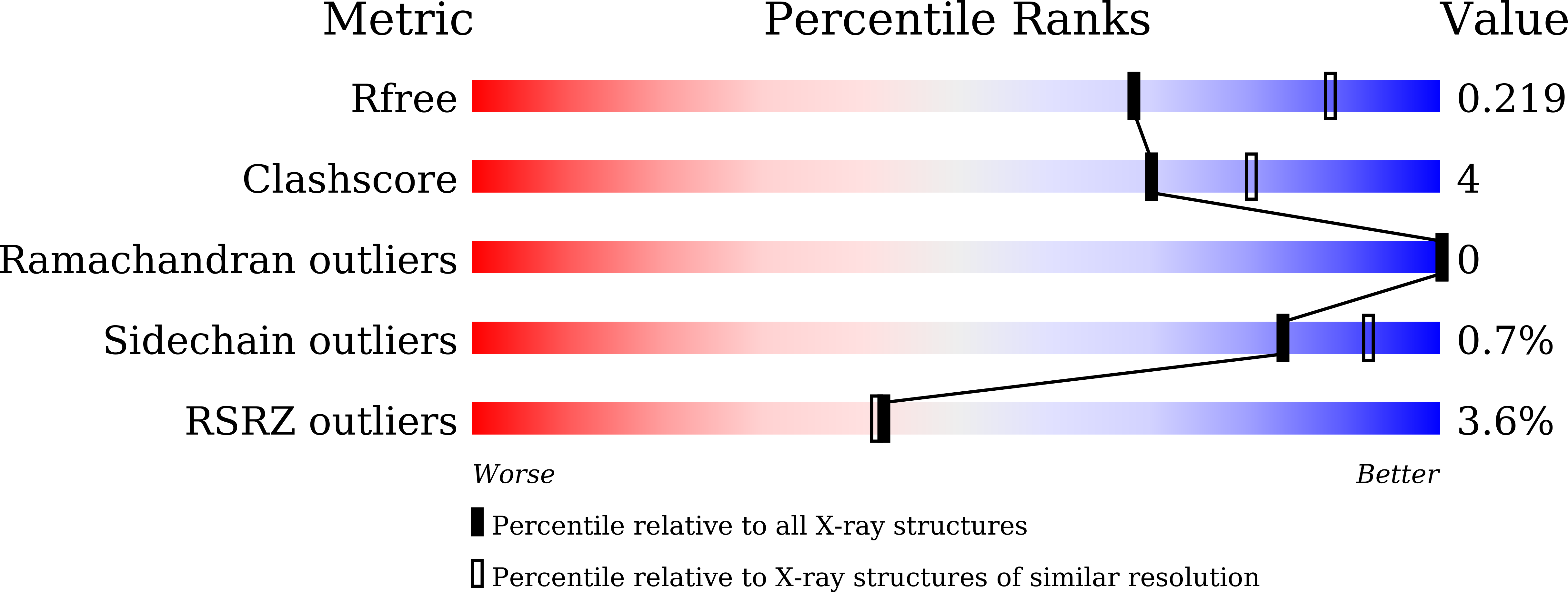
CdpNPT from Aspergillus fumigatus is a fungal indole prenyltransferase (IPT) with remarkable substrate promiscuity to generate prenylated compounds. Our first investigation of the catalytic potential of CdpNPT against a ¦Â-carboline, harmol (1), revealed that the enzyme also accepts 1 as the prenyl acceptor with dimethylallyl diphosphate (DMAPP) as the prenyl donor and selectively prenylates the C-6 position of 1 by the "regular-type" dimethylallylation to produce 6-(3-dimethylallyl)harmol (2). Furthermore, our X-ray crystal structure analysis of the C-His 6 -tagged CdpNPT (38-440) truncated mutant complexed with 1 and docking studies of DMAPP to the crystal structure of the CdpNPT (38-440) mutant suggested that CdpNPT could employ the two-step prenylation system to produce 2.
Organizational Affiliation:
Department of Pharmacognosy, Faculty of Pharmacy, Cairo University, Kasr El-Aini St., Cairo, 11562, Egypt.