Metabolic and Structural Insights into Hydrogen Sulfide Mis-Regulation in Enterococcus faecalis.
Walsh, B.J.C., Costa, S.S., Edmonds, K.A., Trinidad, J.C., Issoglio, F.M., Brito, J.A., Giedroc, D.P.(2022) Antioxidants (Basel) 11
- PubMed: 36009332
- DOI: https://doi.org/10.3390/antiox11081607
- Primary Citation of Related Structures:
8A56 - PubMed Abstract:
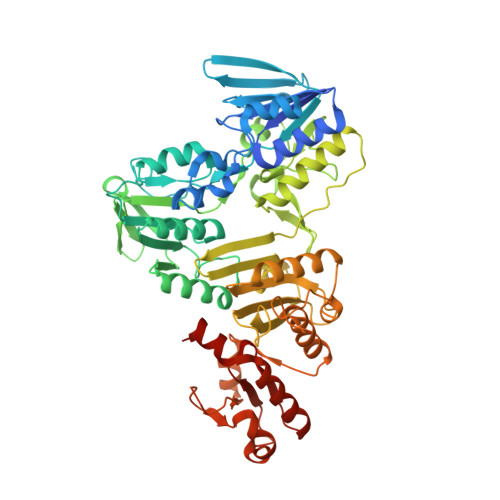
Hydrogen sulfide (H 2 S) is implicated as a cytoprotective agent that bacteria employ in response to host-induced stressors, such as oxidative stress and antibiotics. The physiological benefits often attributed to H 2 S, however, are likely a result of downstream, more oxidized forms of sulfur, collectively termed reactive sulfur species (RSS) and including the organic persulfide (RSSH). Here, we investigated the metabolic response of the commensal gut microorganism Enterococcus faecalis to exogenous Na 2 S as a proxy for H 2 S/RSS toxicity. We found that exogenous sulfide increases protein abundance for enzymes responsible for the biosynthesis of coenzyme A (CoA). Proteome S -sulfuration (persulfidation), a posttranslational modification implicated in H 2 S signal transduction, is also widespread in this organism and is significantly elevated by exogenous sulfide in CstR, the RSS sensor, coenzyme A persulfide (CoASSH) reductase (CoAPR) and enzymes associated with de novo fatty acid biosynthesis and acetyl-CoA synthesis. Exogenous sulfide significantly impacts the speciation of fatty acids as well as cellular concentrations of acetyl-CoA, suggesting that protein persulfidation may impact flux through these pathways. Indeed, CoASSH is an inhibitor of E. faecalis phosphotransacetylase (Pta), suggesting that an important metabolic consequence of increased levels of H 2 S/RSS may be over-persulfidation of this key metabolite, which, in turn, inhibits CoA and acyl-CoA-utilizing enzymes. Our 2.05 ? crystallographic structure of CoA-bound CoAPR provides new structural insights into CoASSH clearance in E. faecalis .
Organizational Affiliation:
Department of Chemistry, Indiana University, Bloomington, IN 47405-7102, USA.