Irreversible inactivation of lactate racemase by sodium borohydride reveals reactivity of the nickel-pincer nucleotide cofactor.
Gatreddi, S., Sui, D., Hausinger, R.P., Hu, J.(2023) ACS Catal 13: 1441-1448
- PubMed: 37886035
- DOI: https://doi.org/10.1021/acscatal.2c05461
- Primary Citation of Related Structures:
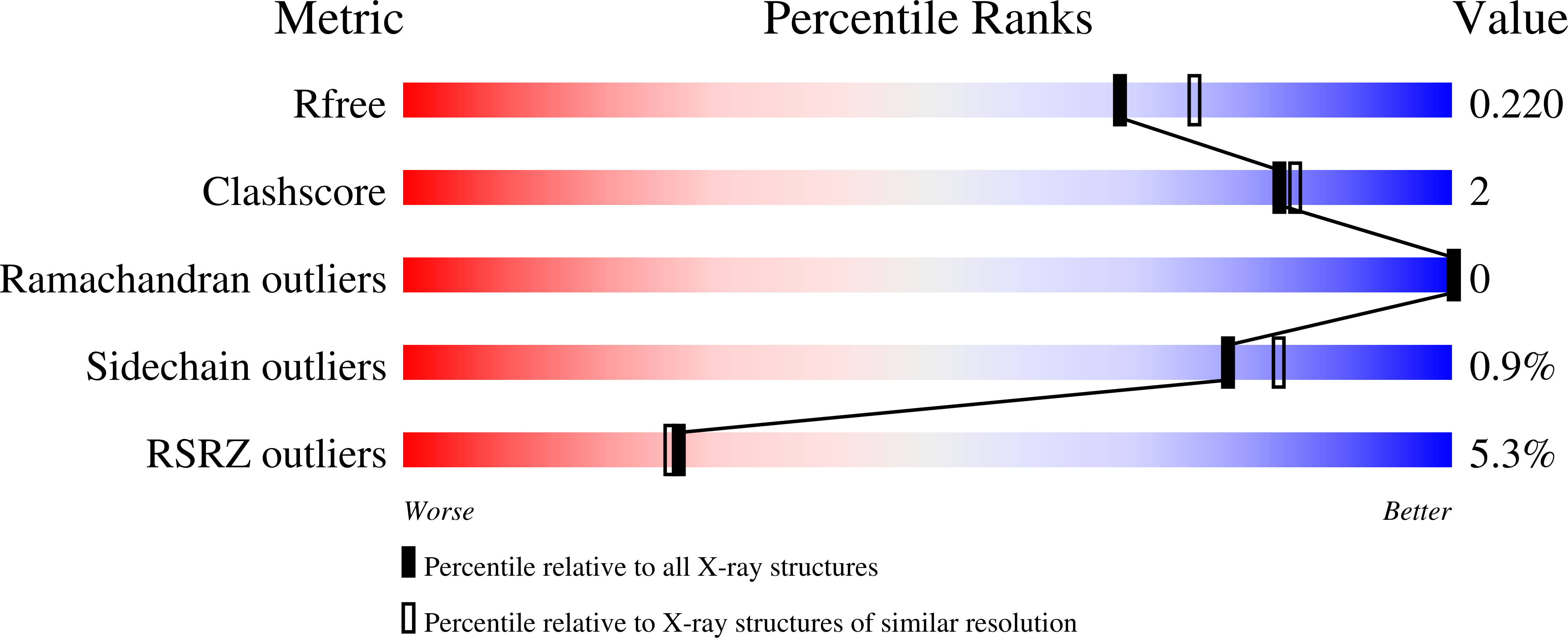
8EZF, 8EZH, 8EZI - PubMed Abstract:
The nickel-pincer nucleotide (NPN) cofactor discovered in lactate racemase from Lactiplantibacillus plantarum (LarA Lp ) is essential for the activities of racemases/epimerases in the highly diverse LarA superfamily. Prior mechanistic studies have established a proton-coupled hydride-transfer mechanism for LarA Lp , but direct evidence showing that hydride attacks the C4 atom in the pyridinium ring of NPN has been lacking. Here, we show that sodium borohydride (NaBH 4 ) irreversibly inactivates LarA Lp accompanied by a rapid color change of the enzyme. The altered ultraviolet-visible spectra during NaBH 4 titration supported hydride transfer to C4 of NPN, and the concomitant Ni loss unraveled by mass spectrometry experiments accounted for the irreversible inactivation. High resolution structures of LarA Lp revealed a substantially weakened C-Ni bond in the metastable sulfite-NPN adduct where the NPN cofactor is in the reduced state. These findings allowed us to propose a mechanism of LarA Lp inactivation by NaBH 4 that provides key insights into the enzyme-catalyzed reaction and sheds light on the reactivity of small molecule NPN mimetics.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Michigan State University, East Lansing, MI 48824, United States.