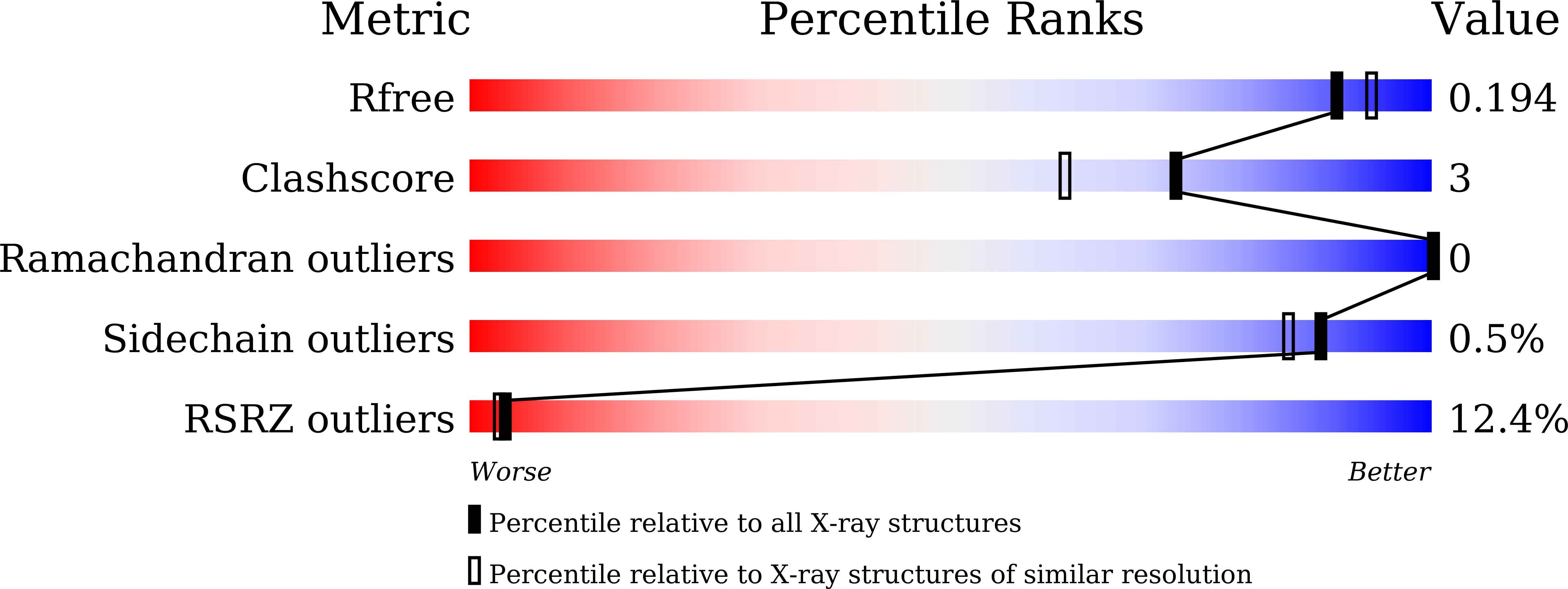
Structures of L-proline trans-hydroxylase reveal the catalytic specificity and provide deeper insight into AKG-dependent hydroxylation.
Hu, X., Huang, X., Liu, J., Zheng, P., Gong, W., Yang, L.(2023) Acta Crystallogr D Struct Biol 79: 318-325
- PubMed: 36974966
- DOI: https://doi.org/10.1107/S2059798323001936
- Primary Citation of Related Structures:
8H7T, 8H7V, 8H7Y, 8H81, 8H85 - PubMed Abstract:
L-Proline hydroxylase is a member of the non-heme Fe 2+ /¦Á-ketoglutarate (AKG)-dependent hydroxylase family that catalyzes the reaction from L-proline to hydroxy-L-proline, which is widely used in drug synthesis, biochemistry, food supplementation and cosmetic industries. Here, the first crystal structure of L-proline trans-hydroxylase and its complexes with substrate and product are reported, which reveal the structural basis of trans-cis proline hydroxylation selectivity. Structure comparison with other AKG-dependent hydroxylases identifies conserved amino acid residues, which may serve as signatures of in-line or off-line AKG binding modes in the AKG-dependent enzyme family.
Organizational Affiliation:
Department of Life Sciences and Medicine, University of Science and Technology of China, Hefei, People's Republic of China.