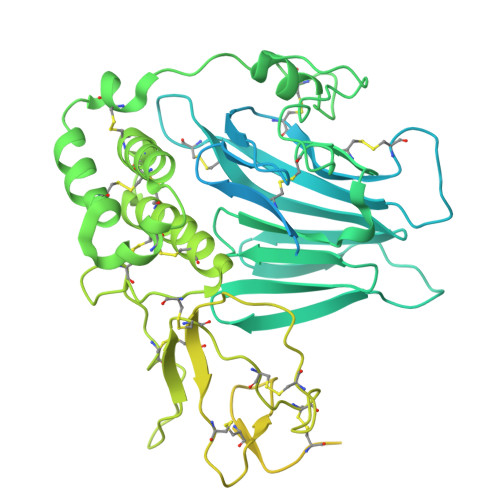
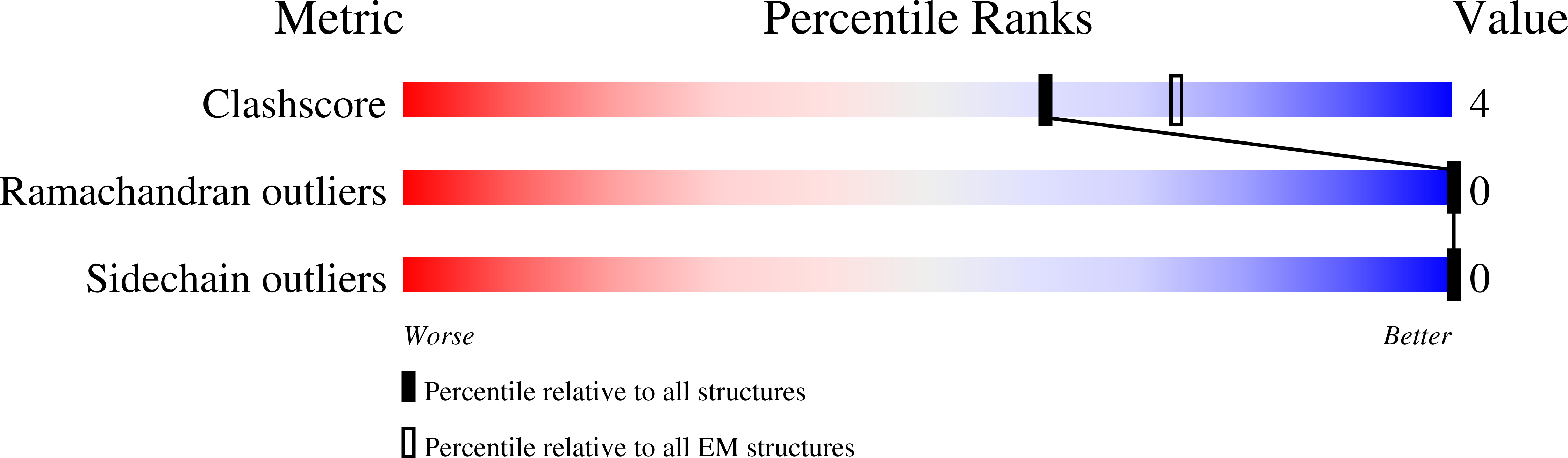Structure of the MUC5AC VWD3 assembly responsible for the formation of net-like mucin polymers.
Trillo-Muyo, S., Ermund, A., Hansson, G.C.(2025) EMBO Rep
- PubMed: 40016425
- DOI: https://doi.org/10.1038/s44319-025-00395-8
- Primary Citation of Related Structures:
8QSP, 8QTB, 8QTV, 8R1U, 8R1Z - PubMed Abstract:
Gel-forming mucins MUC5AC and MUC5B constitute the main structural component of the mucus in the respiratory system. Secreted mucins interact specifically with each other and other molecules giving mucus specific properties. We determined the cryoEM structures of the wild type D3 assembly of the human MUC5AC mucin and the structural single nucleotide polymorphisms (SNP) variants Arg996Gln and Arg1201Trp that affect intermolecular interactions. Our structures explain the MUC5AC N-terminal non-covalent oligomerization after secretion. The D3 assembly forms covalent dimers that can appear in two alternative conformations, open and closed, where the closed conformation dimers interact through an arginine-rich loop in the TIL3 domain to form tetramers. Our study provides a model to explain MUC5AC net-like structures and how the two SNPs will affect mucus organization, something that might affect lung and other diseases.
Organizational Affiliation:
Department of Medical Biochemistry and Cell Biology, University of Gothenburg, 40530, Gothenburg, Sweden. sergio.muyo@medkem.gu.se.