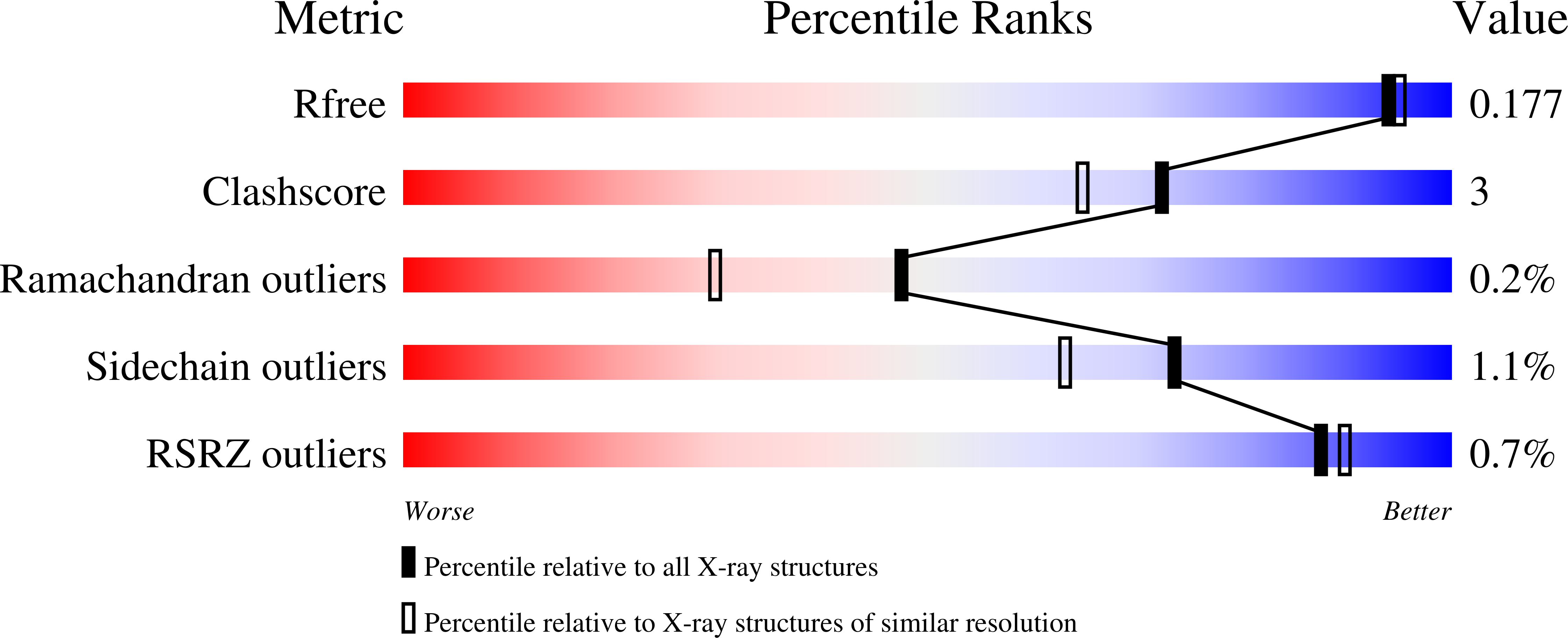
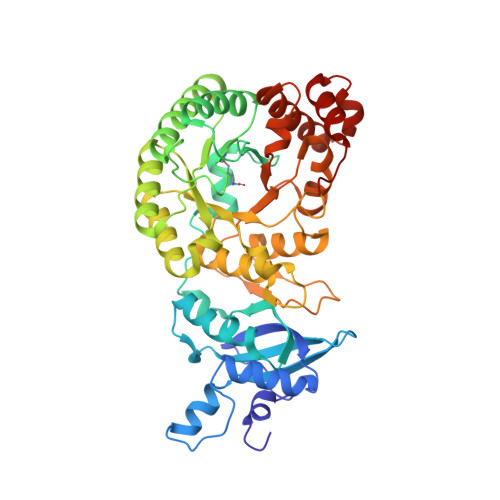
Crystal structure of a type III Rubisco in complex with its product 3-phosphoglycerate.
Huang, Q., Szebenyi, D.M.E.(2023) Proteins 91: 330-337
- PubMed: 36151846
- DOI: https://doi.org/10.1002/prot.26431
- Primary Citation of Related Structures:
8DHT - PubMed Abstract:
The crystal structure of the complex of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco; EC 4.1.1.39) from Archaeoglobus fulgidus (afRubisco) with its products 3PGAs has been determined to a resolution of 1.7?? and is of the closed form. Type III Rubiscos such as afRubisco have 18 out of the 19 essential amino acid residues of canonical Rubisco; the 19th is Tyr rather than Phe. Superposition with the structure of a complex of the similar tkRubisco with the six-carbon intermediate analog 2CABP shows the same conformation of the 19 residues except for Glu46 and Thr51. Glu46 adopts a unique conformation different from that in other Rubiscos and makes two H-bonds with the ligand 3PGA. Similar to other closed state Rubiscos, the backbone of Thr51 is rotated and the side chain makes an H-bond with the ligand 3PGA. Two product 3PGA molecules are bound at the active site, overlapping well with the 2CABP of tkRubisco/2CABP. The positions of the P1 and P2 phosphate groups differ by 0.4 and 0.53??, respectively, between 2CABP and the two 3PGAs. This afRubisco/3PGA complex mimics an intermediate stage of the carboxylation reaction which occurs after the production of the two 3PGA products but before the reopening of the active site. The stability of this complex suggests that the Rubisco active site will not reopen before both 3PGA products are formed.
Organizational Affiliation:
Cornell High Energy Synchrotron Source (CHESS), Cornell University, Ithaca, New York, USA.