Discovery of Elironrasib (RMC-6291), a Potent and Orally Bioavailable, RAS(ON) G12C-Selective, Covalent Tricomplex Inhibitor for the Treatment of Patients with RAS G12C-Addicted Cancers.
Cregg, J., Pota, K., Tomlinson, A.C.A., Yano, J., Marquez, A., Liu, Y., Schulze, C.J., Seamon, K.J., Holderfield, M., Wei, X., Zhuang, Y., Yang, Y.C., Jiang, J., Huang, Y., Zhao, R., Ling, Y., Wang, Z., Flagella, M., Wang, Z., Singh, M., Knox, J.E., Nichols, R., Wildes, D., Smith, J.A.M., Koltun, E.S., Gill, A.L.(2025) J Med Chem 68: 6041-6063
- PubMed: 39993169
- DOI: https://doi.org/10.1021/acs.jmedchem.4c02313
- Primary Citation of Related Structures:
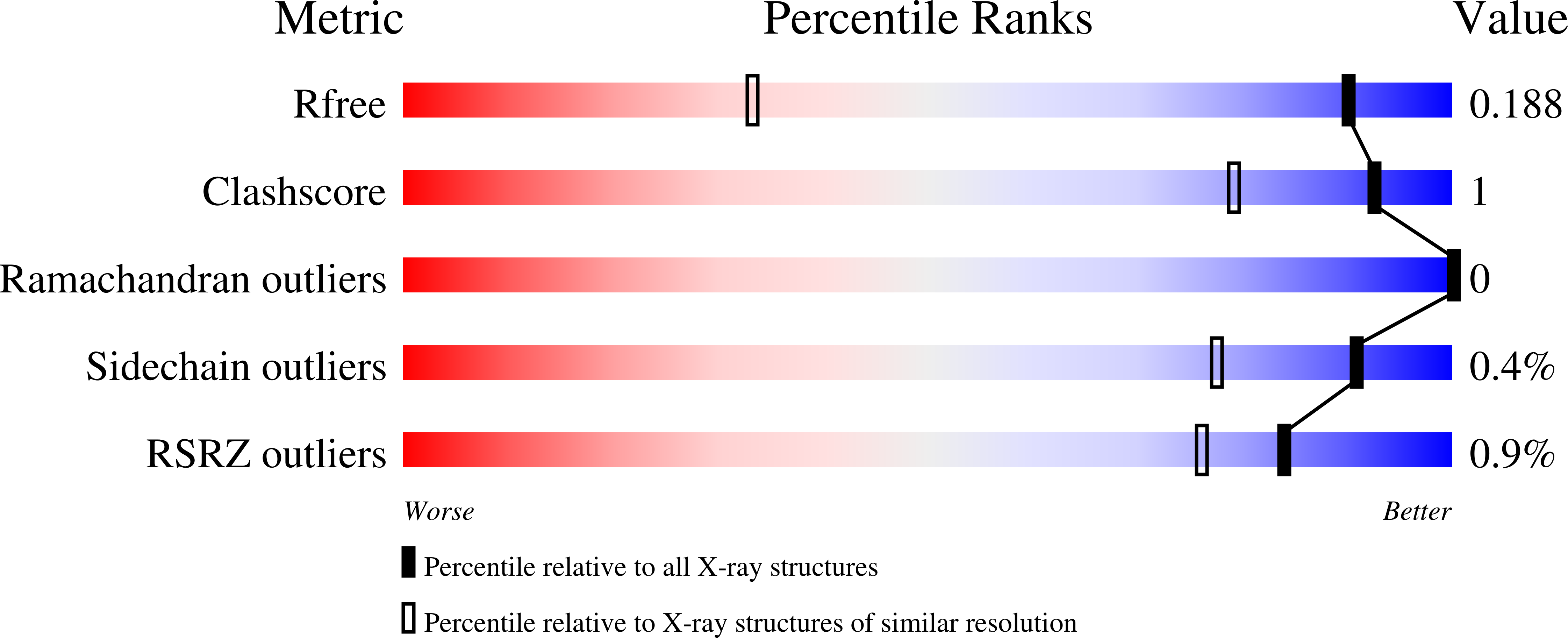
9BFV, 9BFW, 9BFX, 9BFY, 9BFZ - PubMed Abstract:
The discovery of elironrasib (RMC-6291) represents a significant breakthrough in targeting the previously deemed undruggable GTP-bound, active KRAS G12C . To target the active state of RAS (RAS(ON)) directly, we have employed an innovative tri-complex inhibitor (TCI) modality involving formation of a complex with an inhibitor, the intracellular chaperone protein CypA, and the target protein KRAS G12C in its GTP-bound form. The resulting tri-complex inhibits oncogenic signaling, inducing tumor regressions across various preclinical models of KRAS G12C mutant human cancers. Here we report structure-guided medicinal chemistry efforts that led to the discovery of elironrasib, a potent, orally bioavailable, RAS(ON) G12C-selective, covalent, tri-complex inhibitor. The investigational agent elironrasib is currently undergoing phase 1 clinical trials (NCT05462717, NCT06128551, NCT06162221), with preliminary data indicating clinical activity in patients who had progressed on first-generation inactive state-selective KRAS G12C inhibitors.
Organizational Affiliation:
Revolution Medicines, Inc., Redwood City, California 94063, United States.