Transition State Analogs of Human DNPH1 Reveal Two Electrophile Migration Mechanisms.
Wagner, A.G., Lang, T.B.D., Ledingham, E.T., Ghosh, A., Brooks, D., Eskandari, R., Suthagar, K., Almo, S.C., Lamiable-Oulaidi, F., Tyler, P.C., Schramm, V.L.(2025) J Med Chem 68: 3653-3672
- PubMed: 39818772
- DOI: https://doi.org/10.1021/acs.jmedchem.4c02778
- Primary Citation of Related Structures:
9DA1, 9DA2, 9DA3, 9DA4, 9DA5, 9DA6 - PubMed Abstract:
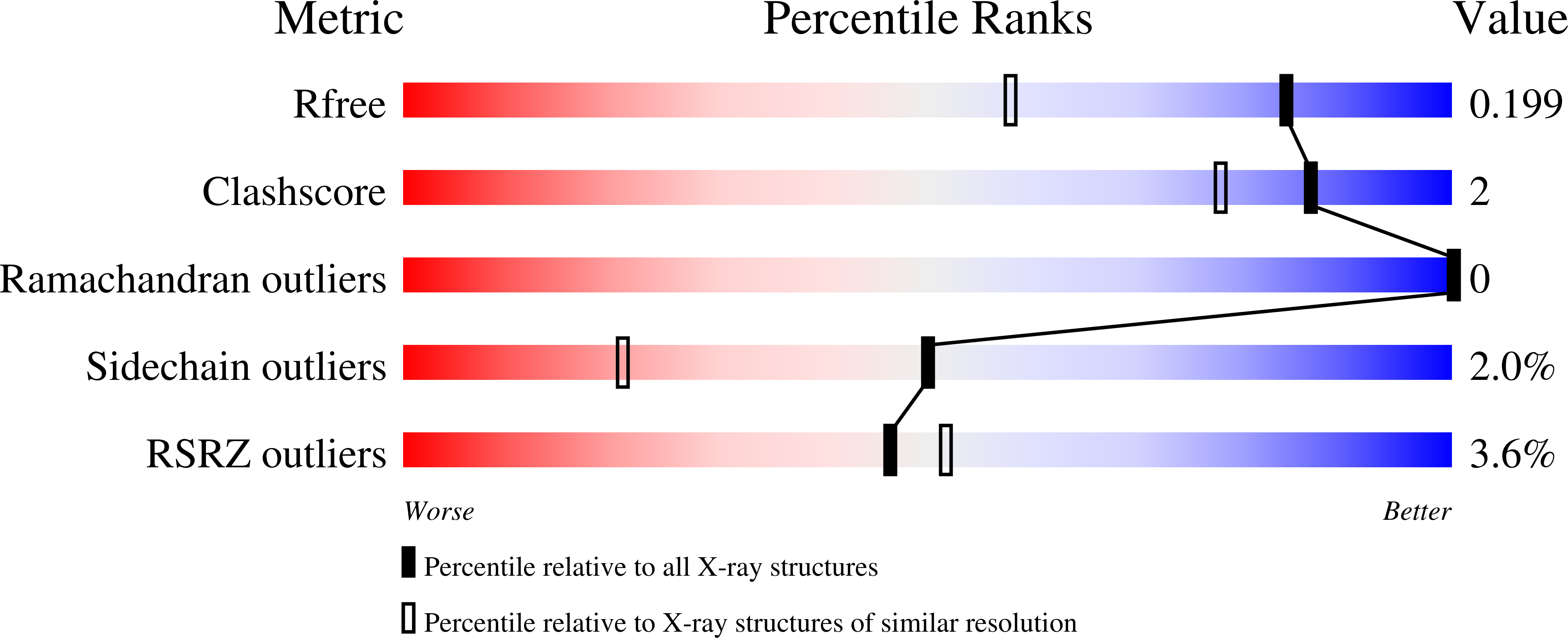
DNPH1 is responsible for eliminating the epigenetically modified nucleotide, 5-hydroxymethyl-2'-deoxyuridine 5'-monophosphate (hmdUMP), preventing formation of hmdUTP, a mutation-inducing nucleotide. Loss of DNPH1 activity sensitizes PARP inhibition-resistant BRCA-deficient cancers by causing incorporation of hmdUTP into DNA. Hydrolysis of hmdUMP by DNPH1 proceeds through a covalent intermediate between Glu104 and 2-deoxyribose 5-phosphate, followed by hydrolysis, a reaction cycle with two transition states. We describe synthesis and characterization of transition state mimics for both transition states of DNPH1. Both transition states prefer inhibitors with cationic charge at the anomeric center and provide a foundation for inhibitor design. Ground-state complexes show reaction coordinate nucleophiles poised 3.3-3.7 ? from the anomeric carbon while transition state analogs tighten the reaction coordinate to place the nucleophiles 2.7-2.8 ? from the anomeric carbon. Crystal structures of DNPH1 with transition state analogs reveal transition states where the electrophilic ribocation migrates between the leaving groups and attacking nucleophiles.
Organizational Affiliation:
Department of Biochemistry, Albert Einstein College of Medicine, Bronx, New York 10461, United States.